-
Medical journals
- Career
Influenza in the pediatric population in Istanbul: a one center experience 2009–2014
Authors: Selda Hançerli Törün 1; Esin Karakılıç 2; Hacer Aktürk 1; Murat Sütçü 1; Metin Uysalol 3; Meral Cıplak 4; Selim Badur 4; Nuran Salman 1; Ayper Somer 1
Authors‘ workplace: Division of Pediatric Infectious Diseases, Istanbul University, Istanbul Faculty of Medicinei Istanbul, Turkey 1; Division of Pediatrics, Istanbul University, Istanbul Faculty of Medicinei Istanbul, Turkey 2; Division of Pediatric Emergency, Department of Pediatrics, Istanbul University, Istanbul Faculty of Medicinei Istanbul, Turkey 3; Virology and Fundamental Immunology Unit, Department of Microbiology and Clinical Microbiology, Istanbul University, Istanbul Faculty of Medicinei Istanbul, Turkey 4
Published in: Epidemiol. Mikrobiol. Imunol. 65, 2016, č. 1, s. 46-50
Category: Original Papers
Overview
Introduction:
The aim of the study was to evaluate the clinical characteristics of pediatric patients with influenza infection.Material and Methods:
The patients hospitalized with confirmed influenza between October 2009 and May 2014 were enrolled in this study.Results:
The mean age of the patients was 66 ± 53 months (1–204 months). Fifty-four percent of patients had a chronic underlying disease. Twenty-four patients needed mechanical ventilation support and a two-month-old patient with liver disease died. Except for the 2009–2010 season, all patients who received mechanical ventilation had underlying disease. The hospital admission months were December-February in 2010–2011 and January-March in 2011-2012 as well as in 2012–2013. Convulsion was observed frequently in influenza A cases, and influenza B tended to be detected in older patients (p = 0.024). The most common symptoms in pediatric patients were fever and cough.Conclusion:
It is obvious that to protect against circulating influenza viruses, the risk-based strategy of annual influenza immunization should target school-aged children and children with underlying conditions, especially neurological and pulmonary diseases.Keywords:
influenza virus – children – clinical characteristicsINTRODUCTION
Each year, about 20% of children worldwide develop symptomatic influenza A and B infections [11]. Children under the age of two and those with underlying medical conditions are at increased risk for hospitalization or severe and complicated influenza infection [6]. The World Health Organization (WHO) tracks influenza virus isolates throughout the world to monitor disease activity and to predict the appropriate components for the annual influenza vaccine.
Young children often pose the greatest challenges for diagnosing and treating the flu because they do not arrive with the full complement of typical symptoms such as fever, mylagias, headaches, malaise, coughs, sore throats and runny noses. But plenty of other respiratory viruses can present in the same way. Influenza surveillance provides valuable data that can guide policy makers in developing programmes to prevent and reduce influenza burden both in adult and pediatric population. The influenza surveillance was initiated in 2003 by now named National Influenza Reference Laboratory, Istanbul Faculty of Medicine.
The type of illness caused by influenza virus depends on the age of the host [13]. This article will first examine epidemiology in hospitalized pediatric population with influenza infection and also cover what is known about influenza vaccination coverage, and treatment in this vulnerable population, with a focus on current recommendations in the Turkey.
MATERIAL AND METHODS
Surveillance data obtained for five consecutive years, 2009–2014, by National Influenza Reference Laboratory in Istanbul Faculty of Medicine have been summarized in this study. The patients who presented to the Istanbul Faculty of Medicine, Pediatrics Emergency Department during October 2009–May 2014 with flu like symptoms and hospitalized patients with confirmed influenza were enrolled in the study.
On admission, nasopharyngeal swabs were collected using Virocult (Medical Wire & Equipment, UK) and along with tracheal aspirates sent to the National Influenza Reference Laboratory located in Istanbul Faculty of Medicine within the same day. All samples were transferred to cryo-tubes upon receiving and stored in -80 °C freezer if not tested on arrival date.
EZ1 Virus mini kit V2.0 (Catalog number: 955134, Qiagen,Germany) was used for total nucleic acid extraction. Real-time PCR based, multiplex FTD® Respiratory Pathogens 21 kit (Fast-track diagnostics Ltd. Malta) was used for detection of respiratory pathogens such as on RotorGene Q platform (Qiagen, Germany).
For detection of Influenza H3 subtype, Influenza B Yamagata and Victoria lineages real-time RT-PCR method was performed using an ABI 7500 platform with CDC primers and probes according to the CDC protocol [15].
Patients’ demographic information was obtained from the hospital records: onset time of disease, age at onset, gender, flu vaccination, underling chronic illness and immunosuppression. Also clinical data for assessment of the disease severity, follow up to discharge or death, length of hospital stay and laboratory data including whole blood count, C-reactive protein (CRP), and detected virus in the nasopharyngeal swap were also obtained.
Oseltamivir was administered to all patients, and antibiotic treatment was added to the patients with suspected secondary bacterial infection who had prolonged fever and elevation of the acute phase reactants. The patients were followed up regularly for the complications of respiratory distress, and the need for mechanical ventilator support.
Clinical characteristics of patients and frequencies of complications were compared using Chi-square test for categorical variables, and medians and distributions of continuous variables were compared using the Student T-test. All analyses were performed using SPSS 21 for Windows was used. P value p < 0.05 was considered significant.
Compliance with Ethical Standards: All authors have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual parents included in the study.
RESULTS
Patients’ characteristics and risk factors
During the study period, 230 pediatric patients (≤ 18 years of age) were enrolled in the study. The mean hospital stay was 6 ± 11 days (1–90 days), and the mean age of the patients was 66 ± 53 months (1–204 months). The percentage of patients 0–3 months of age, 3–12 months of age, 12–24 months of age, 24–60 months of age, 60–120 months of age, > 120 months of age were 3, 14,12.6, 18.6, 27.4, 24.4, respectively (Figure 1). One hundred thirty eight of the patients (n = 138, 60%) were male. The patients presented to the emergency department on an average of 3 ± 2 days (1–12 days), after the onset of the symptoms (Table 1). Convulsion (p = 0.448), myalgia (p = 0.171), diharrea (p = 0.762) where evenly distributed as presentation symptoms regardless of age.
Figure 1. The age distrubition of patients 
1. The symptoms of the patients 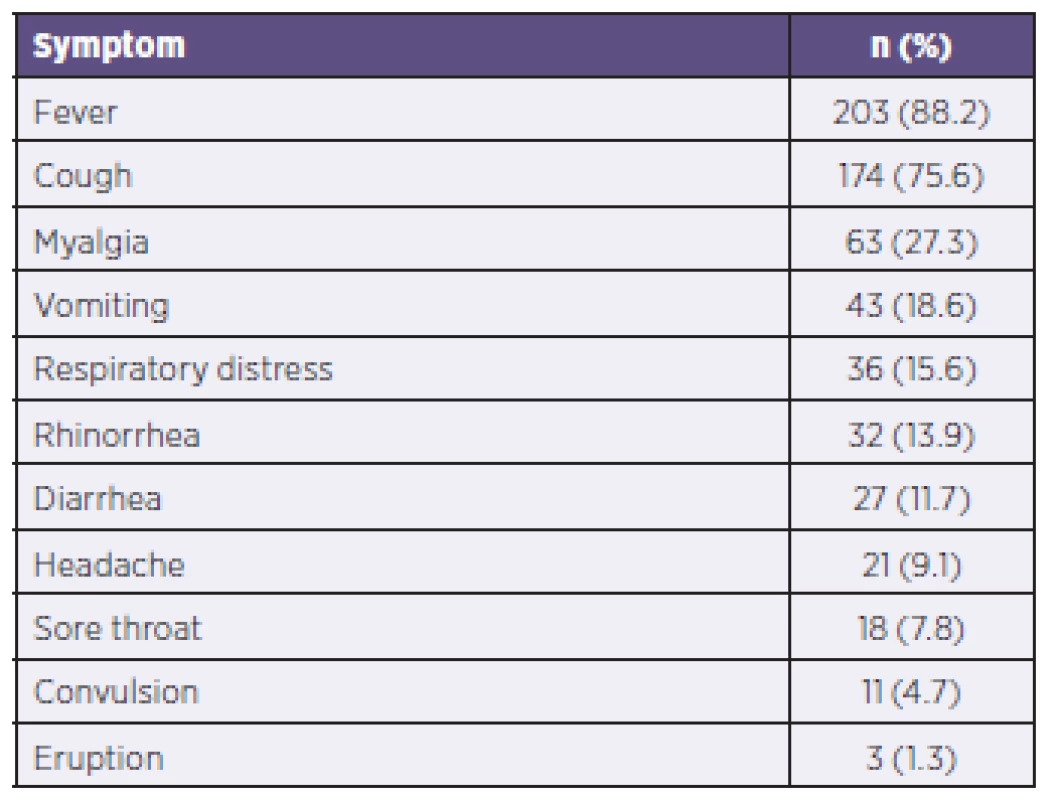
Overall,124 (54 %) of patients had underlying chronic disease and 14% were immunosuppressed. The underlying diseases were asthma (15%), neurological disorders (8.6%), chronic liver-renal disorders (7.2%), primer immunodeficiency disorders(6%), malignity (5.2%), metabolic disease (5.2%), bone marrow/liver/kidney transplantation (3.4%), congenital heart disease (1.8%) and hematological disorders (1.6%).
Of the hospitalized patients, 43 had pneumonia, 34 had acute asthmatic attack, 17 had febrile neutropenia, 6 had status epileptics, 7 had severe myositis, 6 had encephalopathy, 1 had hepatitis and 116 patients had decompansion of underlying disease. Twenty-four patients needed mechanical ventilator support and a two month old patient with liver disease died. Except for 2009–2010 season, all patients who received mechanical ventilation had underlying disease such as neurological and pulmonary disease. Need for mechanical ventilation were higher in patients with no underlying diseases compared to their counter-parts with underlying diseases in 2009–2010.
Season characteristics
The month of patients hospitalized with influenza infection was November-December in 2009–2010 and December-February in 2010–2011, January-March in 2011–2012, and 2012–2013, respectively. During 2013–2014 season influenza viruses circulated the longest year-round with a peak during December-May (Figure 2).
Figure 2. Months of hospitalized patients 
The number of hospitalized patients in 2009–2010, in 2010–2011, in 2011–2012, in 2012–2013 and 2013–2014 were 114, 17, 10, 18 and 71, respectively. Antigenic characteristics of virus and seasonal vaccine components is shown in Table 2. The vaccination rate of patients was 0.8 % (2/230).
2. Influenza subtypes and vaccine covarege 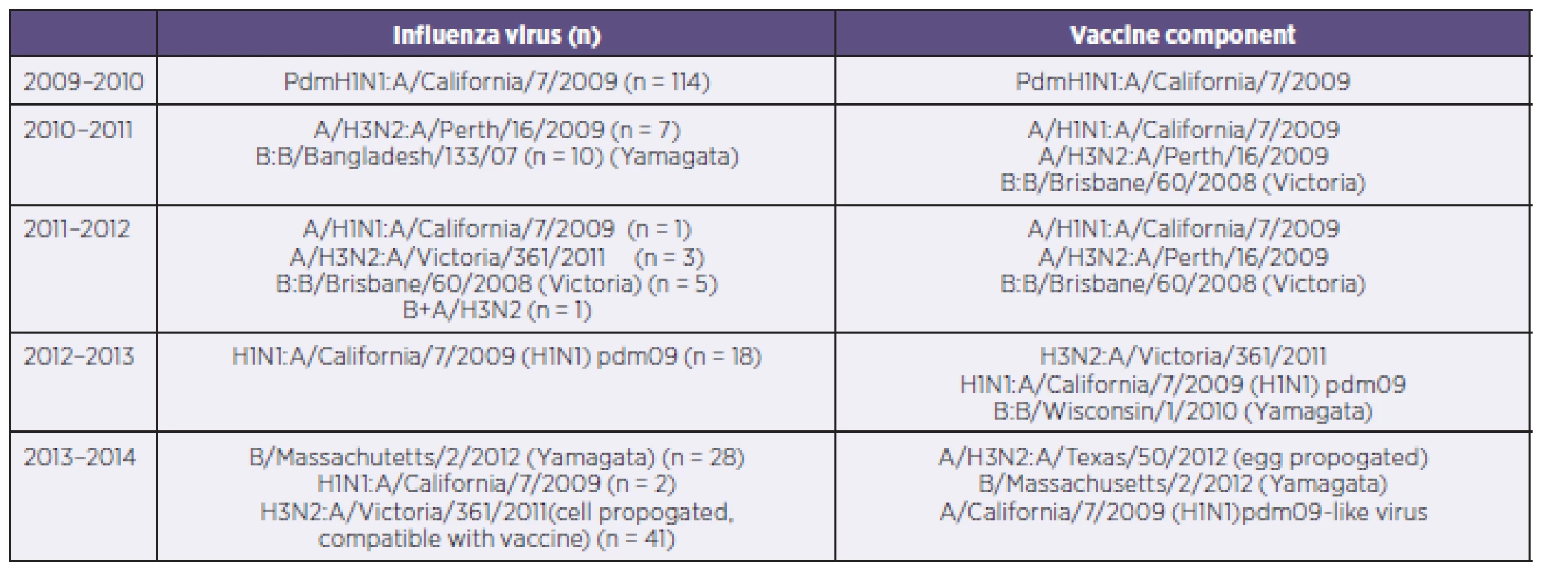
Comparison of patients with influenza A and B
Clinical presentation of influenza B was similar to that of influenza A except convulsion. Convulsion was observed in influenza A cases. Also influenza B tended to be detected in older patients (p = 0.024). Lympopenia was observed in 14 of patients with H3N2, in 12 of patients with influenza B and none of pateints with H1N1 infection. This difference was found statistically significant (p = 0.04). At admission multiple viruses were detected in 7 patients and this did not effect the clinical presentation or prognosis (Table 3).
3. Comparison of patients with influenza A and B 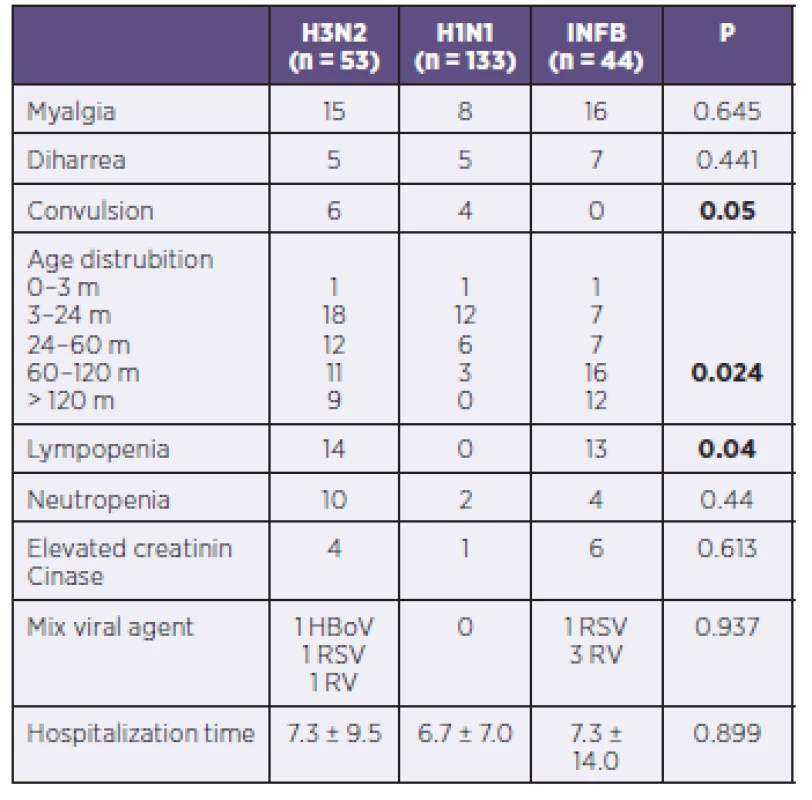
DISCUSSION
Influenza infection is a serious health problem for children and their parents. Influenza spreads around the world in a yearly outbreak, resulting in about three to five million cases of severe illness and about 250,000 to 500,000 deaths [9]. From the 2009–2010 to the 2013–2014 influenza season, in children with laboratory-confirmed influenza identified through a population-based surveillance system, the highest hospitalization rate was for those aged 0–18 years; it was substantially higher in children aged 5–10 years and one hundred thirty eight of the patients (n = 138, 60%) were male. The number hospitalized patients were higher in 2009–2010 and 2013–2014 influenza seaseons than others due to circulating serotype of influenza.
The type of illness caused by influenza virus depends on the age of the host. Signs of respiratory tract involvement are common among preschool and school-age children and Gastrointestinal symptoms are more common in preschool-aged children than in older age [13]. In this study any symptom was not differ from any age distrubition. Children with influenza illness were more likely to have an abrupt onset of fever, cough, myalgia and vomiting. Durig the outbreak of influenza, influenza infection have different manifestations such as diarrhea, convulsion, eruption etc.
Daley’s study showed that there were no significant differences in signs and symptoms of children with influenza A and B virus infection, in severity of illness or in duration of hospital stay and a greater proportion of children admitted with influenza A virus infection were under 12 months of age [3]. In another study it was found that acute childhood myositis was more commonly seen in influenza B infection [8]. In this study we investigated the difference of influenza strains on the clinical presentation. This study showed that Influenza B tended to be detected in older patients. None of patients with influenza B had convulsion and the symptom of convulsion was observed in children with ınfluenza A infection.
It is known that relative lympopenia is typical in older children, and neutrophilia with a left shift occasionally is observed in infants [4]. This study revealed that lympophenia was in 14 of patients with H3N2, in 12 of patients with influenza B and none of patients with H1N1.
The ratio of patients with chronic disease was 54% and with immunsuppresion was 14%. The need for mechanical ventilation and intensive care unit admission was mostly observed in patients with underlying pulmonary and neurological disorders. The Advisory Committe on Immunization Practices of the Centers for Disease Control and Prevention recommends the use of influenza vaccine in persons with conditions that can compromise respiratory function or the handling of respiratory tract secretions or that increase the risk of aspiration [7, 10].
Current guidance recommends that all children who are at higher risk for complications from influenza, which includes all children with neurologic disorders, receive treatment with a neuraminidase inhibitor [5]. Oseltamivir was administered to all patients in this study and mortality rate was very low (1/230 hospitalized patients with influenza). It is important that initiation of antiviral treatment as soon as possible for all patients with confirmed or suspected influenza who have severe complicated or progressive illness or who require hospitalization.
Children with cardiac or pulmonary disease and infants younger than 23 months have heightened risk for severe influenza illness and hospitalization [2]. In this study most of patients (70%) were above 24 months and 51 percents of patients were school-aged children. High infection rates in school-aged children, could be due to prolonged virus shedding in high titers in this age group compared to adults [1]. This data also indicated the importance of vaccination in school children to prevent the spread of the virus hence the epidemics.
Although influenza viruses circulate year-round with a peak during November-April in Northern hemisphere [14], we defined influenza season as December through May in our center. Vaccination coverage (full or partial) by season was 58.8% (10/17) in 2010–2011, 60% (6/10) in 2011–2012, 100% (18/18) in 2012–2013, 100% (56/56) in 2013–2014, respectively. The vaccination rate for influenza is very low in Turkey and also in this study [12]. Vaccination for influenza was generally initiated at October. But the titers of antibody may be decrease until peak of season. As a result of this study we suggest that time of vaccination may be initiated at December.
CONCLUSİONS
Influenza infection is a frequent cause of illness resulting in hospitalization in all children, but is especially common in children above the age of five and those with underlying pulmoner disorders. Typical symptoms among children was fever and cough. Influenza B was more tend to be detected in older patients and convulsion was mostly observed in pateints with influenza A. Annual influenza vaccination is the best method to prevent influenza. Vaccination coverage (full or partial) by five consecutive season was very high. It is clear that to protect against circulating influenza viruses, the risk-based strategy of annual influenza immunization should target school-aged children and children with underlying illness, especially neurological and pulmonary diseases.
Abbreviations
AAP – American Academy Pediatrics
CRP – C-reactive protein
Inf – Influenza virus
WHO – The World Health Organization
Authors’ contribution Dr. Torun conceptualized and designed the study, designed the data collection instruments, coordinated and supervised data collection at the site, carried out the initial analyses, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Karakilic, Dr.Sutcu and Dr. Akturk collected data of the patients. Dr.Uysalol coordinated data collection in the emergency unit. Dr. Cıplak and Dr. Badur analysed samples in the laboratory. Dr. Salman, and Dr Somer critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Acknowledgment: No sources of support, and no conflict of interest.
Conflict of interest: All authors have nothing to disclose of potential conflicts of interest.
Do redakce došlo dne 8. 7. 2015.
Adresa pro korespondenci:
Selda Hançerli Törün, MD
Istanbul University Istanbul Medical Faculty
Department of Pediatric Infectious Diseases Istanbul
Turkey
e-mail: seldahancerli@hotmail.com
Sources
1. Ampofo K, Gesteland PH, Bender J, et al. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics, 2006; 118 : 2409–2417.
2. Committee on Infectious Diseases; American Academy Pediatrics. Recommendations for prevention and control of influenza in children (2014–2015). Pediatrics, 134 : 1503–1519.
3. Daley AJ, Nallusamy R, Isaacs D. Comparison of influenza A and influenza B virus infection in hospitalized children. J Paediatr Child Health, 2000;36(4):332–335.
4. Dawood F, Subbarao K, Fiore A. Influenza viruses. In: Long SS, Pickering LK, Prober CG, (Eds). Principles and Practice of Pediatric Infectious Diseases. 4nd ed. Philadelphia: Churchill Livingstone, 2012, p. 1153–1172.
5. Fiore A, Fry A, Shay D, et al. Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep, 2011;60 : 1–24.
6. Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children – diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis, 2009;48 : 1003.
7. Harper SA, Fukuda K, Uyeki TM, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep, 2005;54 : 1–40.
8. Hu JJ, Kao CL, Lee PI, et al. Clinical features of influenza A and B in children and association with myositis. J Microbiol Immunol Infect, 2004;37 : 95–98.
9. Influenza (Seasonal) Fact sheet. WHO int. March 2014. Retrieved 25 November 2014.
10. Keren R, Zaoutis TE, Bridges CB, et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA,2005; 294 : 2188–2194.
11. Nicholson KG, Wood JM, Zambon M. Influenza. Lancet, 2003;362 : 1733–1745.
12. Meral Ciplak, Grip Platformu. Influenza vaccination in Turkey: Prevalence of risk groups, current vaccination status, factors influencing vaccine uptake and steps taken to increase vaccination rate. Vaccine, 2013;31 : 518–5238.
13. Wang YH, Huang YC, Chang LY, et al. Clinical characteristics of children with influenza A virus infection requiring hospitalization. J Microbiol Immunol Infect, 2003;36 : 111–116.
14. Webster RG, Bean WJ, Gorman OT, et al. Evolution and ecology of influenza A viruses. Microbiol Rev, 1992;56 : 152–179.
15. http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf?ua=1 Accessed: December 23, 2014)
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2016 Issue 1-
All articles in this issue
- The prevalence of Candida pelliculosa, Candida utilis, and Candida fabianii in the Olomouc University Hospital: epidemiological study
- The benefit from mumps virus IgG antibody avidity testing in the population with high vaccine coverage in the context of other serological methods for laboratory diagnosis of mumps and the current epidemiological
- Assessment of invalidity as a result of infectious diseases
- Infectious and other somatic comorbidity in problem drug users – results of a cross-sectional study with medical examination
- Hepatitidis E virus
- Antibiotic treatment of clostridial colitis
- Prevalence of infectious complications in burn patients requiring intensive care: data from a pan-European study
- Influenza in the pediatric population in Istanbul: a one center experience 2009–2014
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Antibiotic treatment of clostridial colitis
- Infectious and other somatic comorbidity in problem drug users – results of a cross-sectional study with medical examination
- Hepatitidis E virus
- Assessment of invalidity as a result of infectious diseases
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

