-
Medical journals
- Career
Metanephric adenoma. A case report and literature review
Authors: Radovan Kaštan 1; Monika Žižlavská 2
Authors‘ workplace: Department of Pathology, Hospital Jihlava, Czech Republic 1; Department of Urology, Hospital Jihlava, Czech Republic 2
Published in: Čes.-slov. Patol., 55, 2019, No. 3, p. 165-169
Category: Original Articles
Overview
Metanephric adenoma is a rare renal tumor with almost exclusively benign behavior, which can clinically and radiologically imitate malignancy. The histological examination is therefore crucial in diagnosis. We report a case of a 69-year-old female with an incidental finding of metanephric adenoma of the left kidney and synchronous clear cell renal cell carcinoma of the contralateral kidney. In the report, we present our experience with this rare tumor and literature review with focusing on differential diagnosis. The histological differential diagnosis of metanephric adenoma includes papillary renal cell carcinoma in adult patients and nephroblastoma (Wilms tumor), particularly in children. Immunohistochemical examination and cytogenetic analyses may be useful in differential diagnosis of these neoplasms.
Keywords:
immunohistochemistry – metanephric adenoma – differential diagnosis – renal tumor
Metanephric adenoma (MA) is a rare renal tumor, which was first time described by Pages and Granier in 1980 as a nephrogenic nephroma (1). MA generally has a benign course, but clinically and radiologically it is difficult to distinguish this neoplasm from other particularly malignant renal tumors. Therefore microscopical diagnosis is crucial. Histological differential diagnosis includes nephroblastoma or Wilms tumor (WT), particularly in children and papillary renal cell carcinoma (PRCC) in adult patients. Especially histological appearances of the epithelial-predominant WT and solid variant of PRCC may be very similar to those of MA. In these cases, the immunohistochemical (IHC) examination and cytogenetic analyses may be useful. MA is usually unilateral solitary tumor and only rare cases of simultaneous diagnosis of MA with another different renal tumor were described. We report a case of the 69-year-old female with metanephric adenoma of the left kidney and synchronous clear cell renal cell carcinoma of the right kidney. Both of these tumors were revealed incidentally during the radiological examination of left ovary tumor. Another goal is to present the literature review of MA with focusing on morphological and immunohistochemical characteristics and histological differential diagnosis of this rare tumor.
CLINICAL HISTORY
The 69-year-old woman presented with diabetes mellitus, arterial hypertension, and ischemic heart disease was admitted to the Oncogynecology center of Hospital in Jihlava because of a huge left ovary tumor suspicious of cystadenoma. An ultrasound examination (USG) and computerized tomography scan (CT) of abdominal cavity were performed. Both radiological examinations revealed a huge cystic left ovarian tumor. Moreover, the radiological examination detected incidental findings of a well-circumscribed low-echo tumor 2cm in diameter on the convexity of the left kidney, which was suspicious of malignancy and multicystic lesion 10,8cm in diameter of the right kidney. The patient underwent hysterectomy with adnexectomy and the ovarian tumor was histologically diagnosed as a mucinous cystadenoma. Three months later the patient underwent partial nephrectomy of the left kidney. Because of a discrete progression of the right kidney cystic lesion on repeated CT imaging patient underwent complete nephrectomy of right kidney seven months after partial left nephrectomy.
MATERIALS AND METHODS
Sections from formalin-fixed, paraffin-embedded blocks were stained with hematoxylin-eosin (HE). Immunohistochemical stainings were performed using antibodies directed against the following antigens: cytokeratin cocktail (AE1/AE3), cytokeratin 7 (CK7), alpha methylacyl coenzyme A racemase (AMACR), Wilms’ tumor-1 protein (WT-1), CD57, epithelial membrane antigen (EMA) and vimentin (VIM). The characteristics of immunohistochemical antibodies (the clone, dilutions, and source) are listed in Table 1. Positive and negative controls gave appropriate results for each procedure.
1. The list of used antibodies. Antibodies Clone Dilution 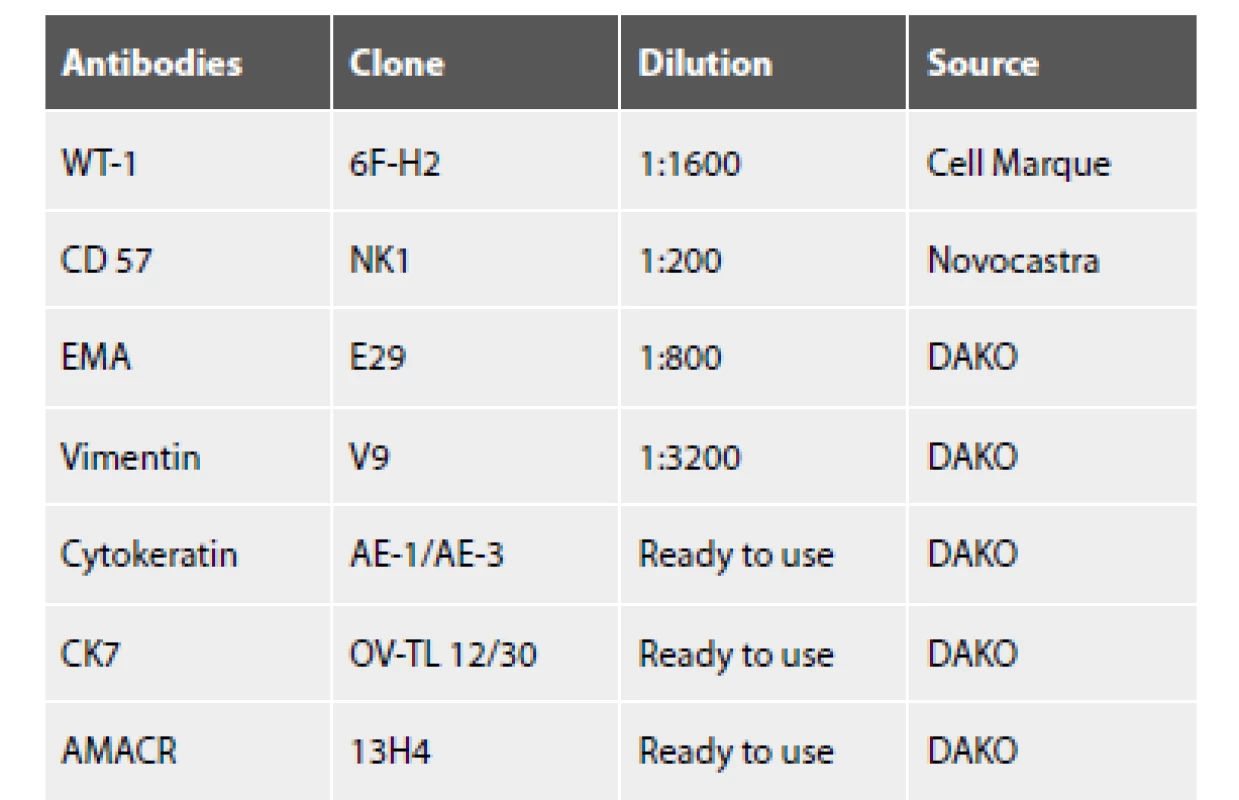
WT-1 - Wilms’ tumor 1, CD – cluster of differentiation, EMA - epithelial membrane antigen, CK – cytokeratin, AMACR - alpha methylacyl coenzyme A racemase RESULTS
Grossly, the well-circumscribed tumor measuring 2,5cm in diameter was present in the partial left nephrectomy specimen. On the cut surface, the tumor was homogenous, tan to yellow in color. Microscopically, the tumor was unencapsulated and sharply demarcated from the surrounding renal parenchyma (Fig.1). The tumor was composed predominantly of small round to oval tubules and acinar structures with minimal stroma (Fig.1,2). Focally a tubulopapillary structures (Fig. 3) and glomeruloid bodies (Fig. 4) were apparent. The tumor cells were tightly packed, small, uniform with the hyperchromatic round to oval partially overlapping nuclei and scant cytoplasm. No mitoses were visible. Nucleoli were inapparent (Fig. 5). Psammoma bodies, calcification or necrosis were not found. All surgical margins were negative. Immunohistochemically, the tumor cells exhibited diffuse positivity for WT1, CD57, vimentin, and cytokeratin cocktail AE1/AE3. IHC examinations for EMA, AMACR, and CK7 were negative (Fig. 6). Based on these findings, a diagnosis of metanephric adenoma was stated. Cytogenetic analysis was not performed.
1. MA is unencapsulated and sharply demarcated from the surrounding renal parenchyma (HE, 100x). 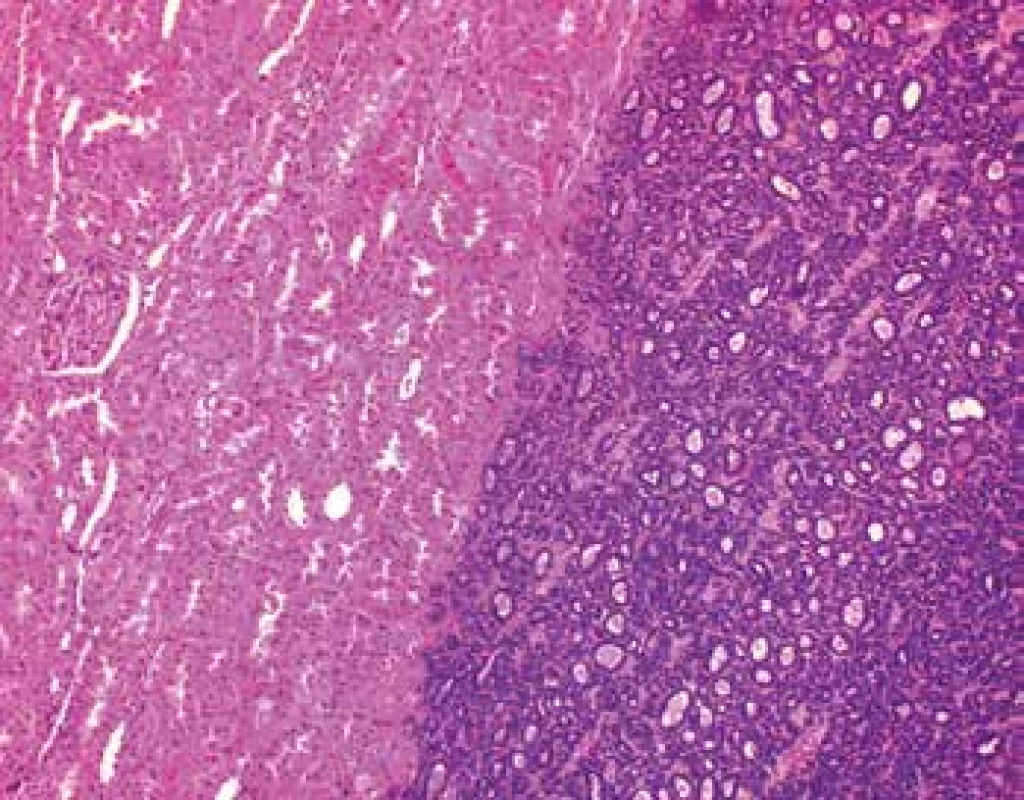
2. Tumor is composed predominantly of tightly packet tubules and acinar structures with minimal stroma (HE, 200x). 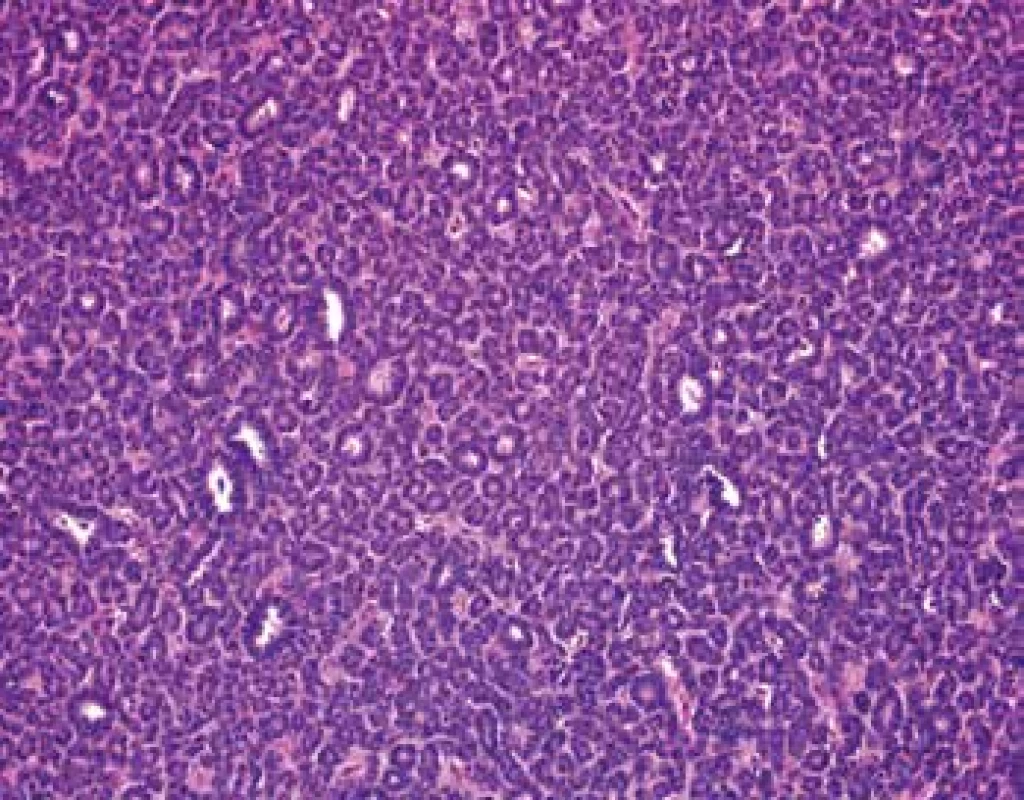
3. In some areas is tumor composed of tubulopapillary structures (HE, 100x). 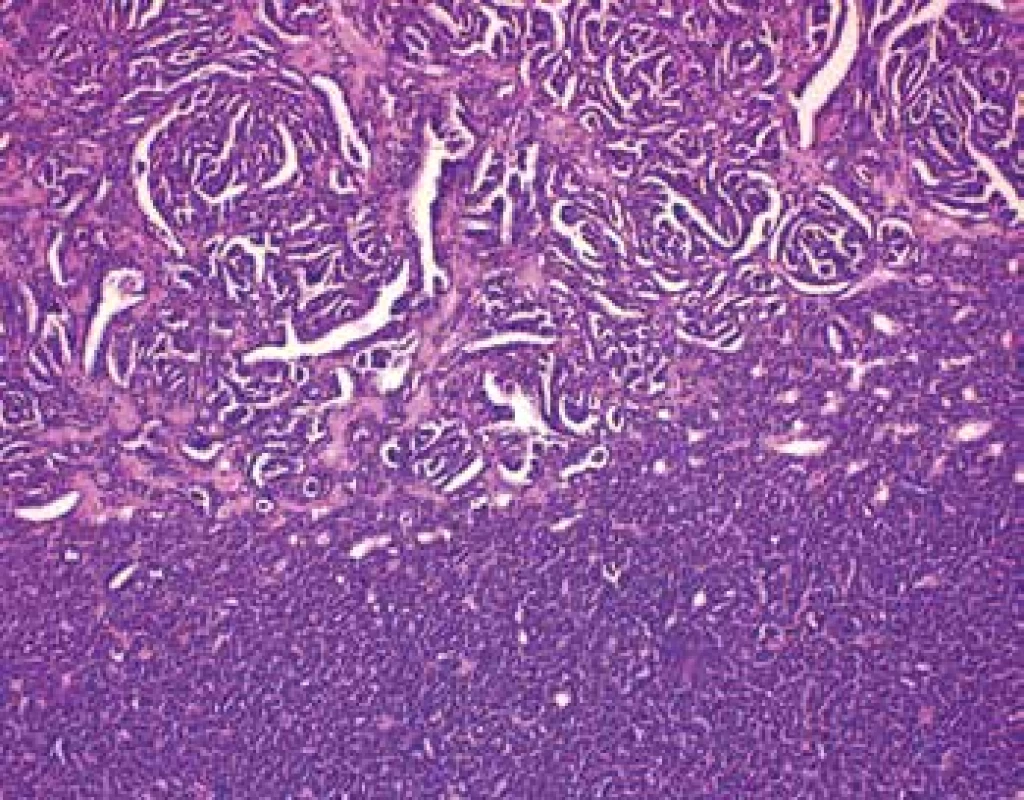
4. Focally a glomeruloid bodies are apparent (HE, 200x). 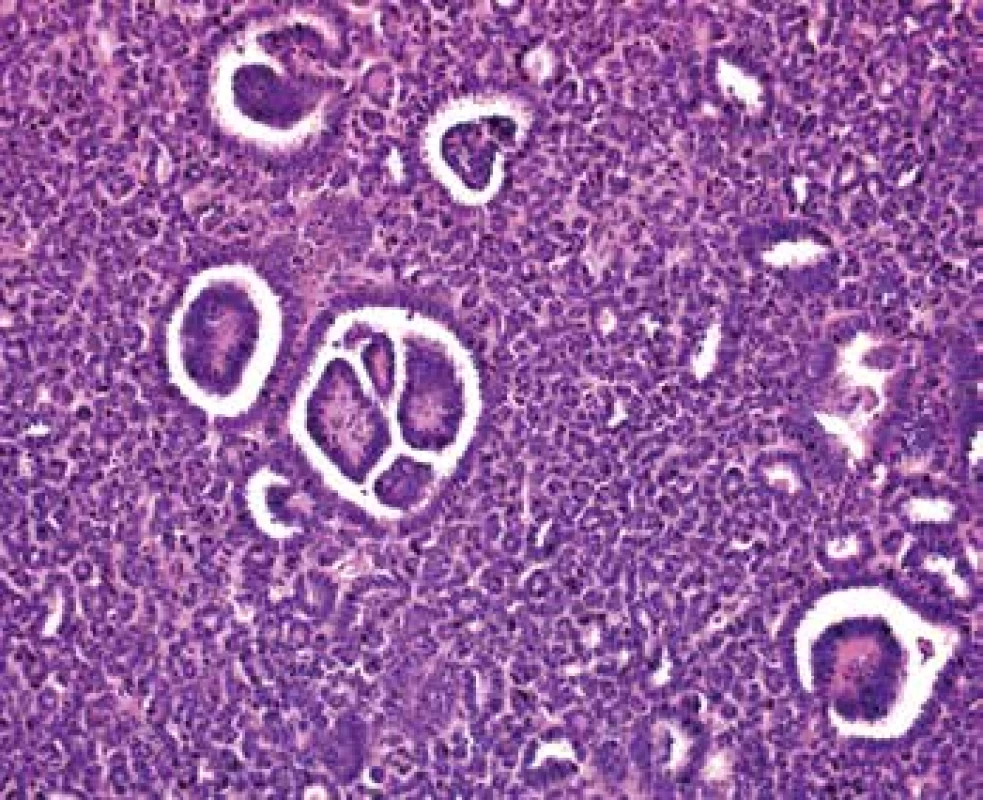
5. Tightly packed small uniform tumor cells with the hyperchromatic round to oval partially overlapping nuclei and scant cytoplasm. Nucleoli and mitotic figures are not apparent (HE, 630x). 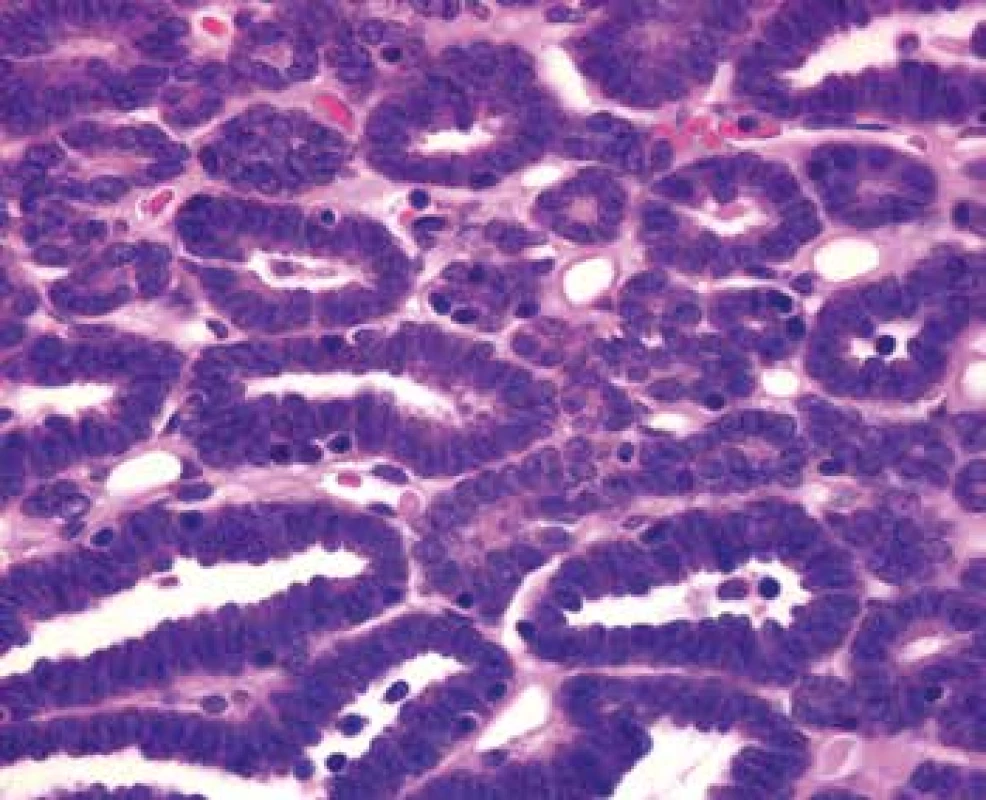
6. Expression of IHC markers: A. WT1 nuclear expression. Positive control – glomerulus in the adjacent renal parenchyma (200x). B. VIM cytoplasmatic expression (400x). C. CD57 cytoplasmatic expression (400x). D. Negativity for AMACR in tumor cells. Positive control – kidney proximal tubules (400x). E. Negativity for EMA in tumor cells. Positive control – kidney distal tubules (400x). F. Negativity for CK7 in tumor cells. Positive control – kidney collecting ducts (400x). 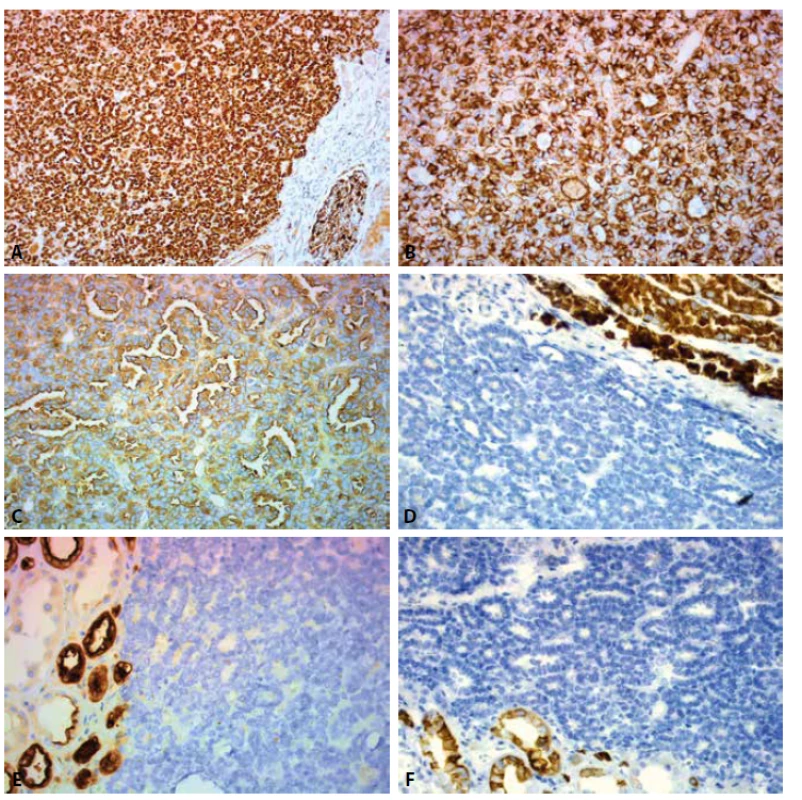
The examination of right kidney revealed partially cystic and partially solid tumor of upper pole measuring 10,8cm in diameter. Microscopic examination showed moderately differentiated clear cell renal cell carcinoma with cystic changes. Infiltration of extrarenal tissues and angioinvasion were not present.
The patient was followed up for 22 months after partial left nephrectomy without signs of local tumor recurrence and metastasis.
DISCUSSION
MA is a rare epithelial tumor of the kidney, which accounts 0,2% of renal tumors (2). Clinicopathological characteristics of MA are obtained from few previously published series. The mean age of the patients was 41-51 years (range of 5 to 84 years) in reported studies (3-7). The females are affected more common than males with female-to-male ratio 2 : 1 – 9,5 : 1. (3,5-7). The size of MA ranges of 0,3 to 15cm (3,6). MA generally has a benign course. It was described only occasional case with lymph node metastases (8). Some authors consider the lymph node involvement as a result of passive mechanical seeding of tumor cells into the lymph node and not as a manifestation of metastatic disease (9). Approximately half of the patient have no clinical symptoms and the tumor is diagnosed incidentally. Symptomatic patients may present with microscopic haematuria, polycythaemia, arterial hypertension, palpable mass and flank of abdominal pain (3,5). Polycythaemia was associated with MA in 12%. (3). The tumor is solitary in the majority of cases, although multifocal and bilateral lesions have been reported (10,11). Very rare case reports of composite tumors with foci of MA and different malignant components or simultaneous MA and another renal tumor in different part of the same kidney or contralateral kidney have been reported (11-13). Grossly, MA is well circumscribed, soft or hard tumor with homogeneously gray to tan to yellow cut surface. Secondary changes like cyst formation, hemorrhage or necrosis may be present in large tumors. Calcification may be apparent macroscopically and may be extensive. Microscopically, the tumor is well circumscribed and composed of small uniform epithelial cells, that form small acinar and tubular formations, less frequently has a papillary architecture with glomeruloid bodies. The stroma is hypocellular and may be oedematous or hyalinized. The frequently overlapping tumor cells have scant pale cytoplasm, and round to oval hyperchromatic nuclei with inconspicuous nucleoli. Mitotic figures are rare or absent. Psammoma bodies and dystrophic calcification are frequently seen. Most tumors are unencapsulated or have the pseudocapsule only (3,14,15). In our case, macroscopic and microscopic findings of MA were consistent with cases reported previously. The differential diagnosis of renal MA includes WT, particularly in children and PRCC (mainly solid variant) in adult patients. Histologically, there is characteristic triphasic pattern for WT (including the epithelial, mesenchymal and blastemal component having high grade nuclear features) and significant mitotic activity in comparison to MA. PRCC usually have a thick fibrous pseudocapsule and occasional cells with abundant cytoplasm and nuclei with vesicular chromatin and variably prominent nucleoli in comparison to MA. Morphology of epithelial-predominant type of WT and the solid variant of PRCC may be similar to those of MA. IHC allows distinguishing MA from the two mentioned malignant neoplasms. The most useful IHC markers in differential diagnosis of MA, PRCC and WT are WT1, CD57, EMA, AMACR, RCC, CK7, S100. According some more recent studies the antibody cadherin 17 is a sensitive and highly specific marker in distinguishing MA from its mimics. In one study 81% of MA demonstrated membranous positivity, whereas all cases of WT and PRCC were negative (20). The typical IHC profiles of these tumors are listed in Table 2. In the majority of cases, a diagnosis can be stated by routine HE staining and by IHC analyses. In the most diagnostically challenging cases, a molecular analysis can be performed. A study of 29 MA and 129 non-MA renal neoplasms revealed, that approximately 90% of MA harbor BRAF V600E mutations, whereas similar mutations are exceedingly rare in other renal tumors (21). A recent study validated, that also positive IHC staining for BRAF V600E supports the diagnosis of MA. However, the absence of BRAF V600E staining does not exclude the possibility of MA (22). The accurate histological diagnosis of MA is important because of difficult clinical and radiological diagnosis of this tumor and different prognosis in comparison to other microscopically similar malignant neoplasms.
2. The most useful immunohistochemical antibodies in the differential diagnosis of metanephric adenoma, papillary renal cell carcinoma, and Wilms tumor (6-7,16-20). 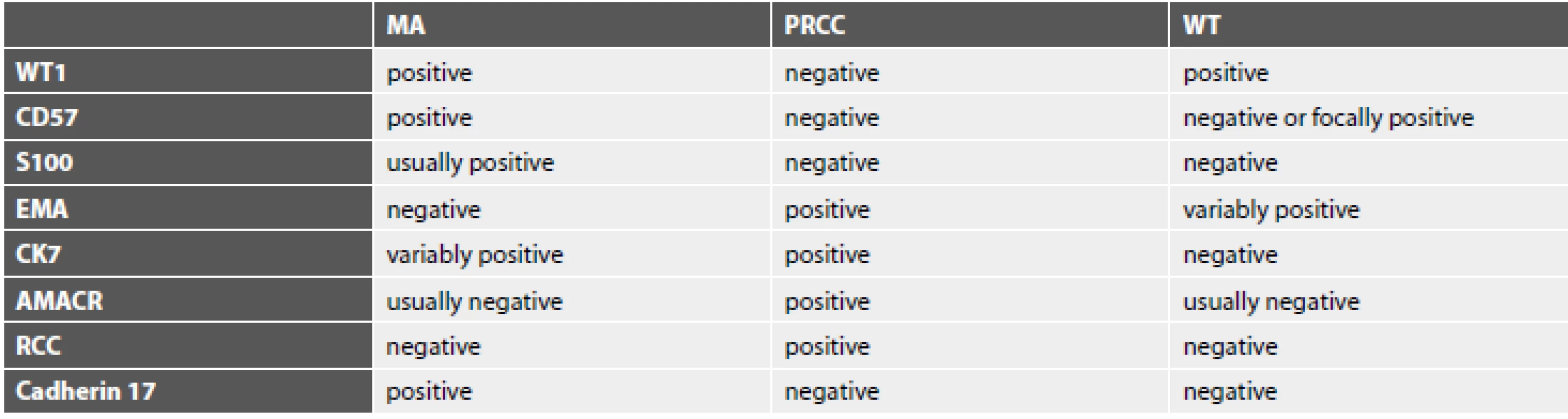
MA – metanephric adenoma, PRCC – papillary renal cell carcinoma, WT – Wilms tumor, WT-1 - Wilms’ tumor 1, CD – cluster of differentiation, EMA - epithelial membrane antigen, CK – cytokeratin, AMACR - alpha methylacyl coenzyme A racemase, RCC – renal cell carcinoma marker ACKNOWLEDGMENTS
We acknowledge MUDr. Eva Krejčí and prim. MUDr. Pavel Fabian, Ph.D. (Department of Oncological Pathology, Masaryk Memorial Cancer Institute, Brno, Czech Republic) for their contribution to this article.
CONFLICT OF INTEREST
The authors declare that there is not a conflict of interest regarding the publication of this paper.
Correspondence address:
MUDr. Radovan Kaštan,
Hospital Jihlava
Vrchlického 59, 586 33 Jihlava, Czech Republic
phone: +420722369538
e-mail: kastanr@nemji.cz
Sources
1. Pages A, Granier M. Nephrogenic nephroma. Arch Anat Cytol Pathol 1980; 28(2): 99-103.
2. Amin MB, Tamboli P, Javidan J, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 2002; 26(3): 281-291.
3. Davis CJ Jr, Barton JH, Sesterhenn IA, Mostofi FK. Metanephric adenoma. Clinicopathological study of fifty patients. Am J Surg Pathol 1995; 19(10): 1101-1114.
4. Hes O, Michal M, Perez-Montiel DM, et al. Metanefrický adenom. Popis 11 případů s průkazem kolagenních sferul v jednom nádoru. Cesk Patol 2002; 38 : 101-106.
5. Bastide C, Rambeaud JJ, Bach AM, Russo P. Metanephric adenoma of the kidney: clinical and radiological study of nine cases. BJU Int 2009; 103(11): 1544-1548.6. Kinney SN, Eble JN, Hes O, et al. Metanephric adenoma: the utility of immunohistochemical and cytogenetic analyses in differential diagnosis, including solid variant papillary renal cell carcinoma and epithelial-predominant nephroblastoma. Mod Pathol 2015; 28(9): 1236-1248.
7. Mantoan Padilha M, Billis A, Allende D, Zhou M, Magi-Galluzzi C. Metanephric adenoma and solid variant of papillary renal cell carcinoma: common and distinctive features. Histopathology 2013; 62(6): 941-953.
8. Renshaw AA, Freyer DR, Hammers YA. Metastatic metanephric adenoma in a child. Am J Surg Pathol 2000; 24(4): 570-574.
9. Paner GP, Turk TM, Clark Ji, Lindgren V, Picken MM. Passive seeding in metanephric adenoma: a review of pseudometastatic lesions in perinephric lymph nodes. Arch Pathol Lab Med 2005; 129(10): 1317-1321.
10. Kohashi K, Oda Y, Nakamori M, et al. Multifocal metanephric adenoma in childhood. Pathol Int 2009; 59(1): 49-52.
11. Pasricha S, Gandhi JS, Gupta G, Mehta A, Beg S. Bilateral, multicentric metanephric adenoma associated with Wilms‘ tumor in child: a rare presentation with important diagnostic and therapeutic implications. Int J Urol 2012; 19(12): 1114-1117.
12. Gang L, Yuhong T, Renya Z, Hualin S, Shumin Z, Yuanjie N. Adult metanephric adenoma presumed to be all benign? A clinical perspective. BMC Cancer 2015; 15 : 310.
13. Anastasiadis AG, Ebert T, Gerharz CD, Ackermann R. Simultaneous diagnosis of a metanephric adenoma and a clear cell carcinoma of the contralateral kidney. Eur Urol 2001; 39(2): 236-239.
14. Tsuji M, Murakami Y, Kanayama H, Sano T, Kagawa S. A case of renal metanephric adenoma: histologic, immunohistochemical and cytogenetic analyses. Int J Urol 1999; 6(4): 203-207.
15. Obulareddy SJ, Xin J, Truskinovsky AM, Anderson JK, Franklin MJ, Fudek AZ. Metanephric adenoma of the kidney: an unusual diagnostic challenge. Rare Tumors 2010; 30 : 2(2): e38 : 103-105.
16. Olgac S, Hutchinson B, Tickoo SK, Reuter VE. Alpha-methylacyl-CoA racemase as a marker in the differential diagnosis of metanephric adenoma. Mod Pathol 2006; 19(2): 218-224.
17. Chen L, Deng FM, Melamed J, Zhou M. Differential diagnosis of renal tumors with tubulopapillary architecture in children and young adults: a case report and review of literature. Am J Clin Exp Urol 2014; 2(3): 266-272.
18. Sun Z, Kan S, Zhang L, et al. Immunohistochemical phenotype and molecular pathological characteristics of metanephric adenoma. Int J Clin Exp Pathol 2015; 8(6): 6031-6036.
19. Kuroda N, Tanaka A, Ohe C, Nagashima Y. Recent advances of immunohistochemistry for diagnosis of renal tumors. Pathol Int 2013; 63(8): 381-390.
20. Yakirevich Y, Magi-Galluzzi C, Grada Z, Lu S, Resnick MB, Mangray S. Cadherin 17 is a sensitive and specific marker for metanephric adenoma. Am J Surg Pathol 2015; 39(4): 479-486.
21. Choueiri TK, Cheville J, Palescandolo E, et al. BRAF mutations in metanephric adenoma of the kidney. Eur Urol 2012; 62(5):917-922.
22. Udager AM, Pan J, Magers MJ, et al. Molecular and immunohistochemical characterization reveals novel BRAF mutations in metanephric adenoma. Am J Surg Pathol 2015; 39(4): 549-557.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2019 Issue 3-
All articles in this issue
- Monitor aneb nemělo by vám uniknout, že...
- Pituitary adenomas – practical approach to the diagnosis and the changes in the 2017 WHO classification
- Cytological examination of cerebrospinal fluid
- Histopathological assessment of the intensity and activity of the inflammation in inflammatory bowel diseases: An important addition to the endoscopy, or a pointless effort?
- The changes of angiogenesis and immune regulations in stromal microenvironment of cutaneous melanomas
- Neuronal ceroid lipofuscinosis with cardiac involvement
- Metanephric adenoma. A case report and literature review
- Atypical fibroxanthoma, rare and often unrecognized cutaneous soft tissue tumor – a case report and review of the literature
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Cytological examination of cerebrospinal fluid
- Neuronal ceroid lipofuscinosis with cardiac involvement
- Pituitary adenomas – practical approach to the diagnosis and the changes in the 2017 WHO classification
- Atypical fibroxanthoma, rare and often unrecognized cutaneous soft tissue tumor – a case report and review of the literature
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

