-
Medical journals
- Career
Pyloric gland adenoma: a histologic, immunohistochemical and molecular genetic study of 23 cases
Authors: Alena Chlumská 1,2; Tomáš Waloschek 1; Petr Mukenšnabl 1,2; Petr Martínek 1; Jana Kašpírková 1; Michal Zámečník 3,4
Authors‘ workplace: Laboratory of Surgical Pathology, s. r. o., Pilsen, Czech Republic 1; Šikl`s Department of Pathology, Faculty Hospital in Pilsen, Charles University in Prague, Pilsen, Czech Republic 2; Department of Pathology, AGEL Laboratories, a. s., Nový Jičín, Czech Republic 3; Medirex Group Academy, n. o., Bratislava, Slovak Republic 4
Published in: Čes.-slov. Patol., 51, 2015, No. 3, p. 137-143
Category: Original Article
Overview
Pyloric gland adenoma is a rare neoplasm with a gastric epithelial differentiation. We report 23 cases of pyloric gland adenoma in older persons, with a mean age of 74 years (range 52 - 87 years). They occurred in the esophagus (3 cases), corporal gastric mucosa (7 cases), duodenum (10 cases), gallbladder (2 cases), and choledochus (one case). Histologically, they were characterized by closely packed pyloric gland-type tubules with a monolayer of cuboidal to low columnar epithelial cells containing basally located round nuclei, and a superficial layer of tall, columnar, foveolar-type epithelium. Immunohistochemically, most tumor glands expressed pyloric gland mucin MUC6, whereas MUC5AC was positive in superficial gastric foveolar epithelium, and in a minority of glands. In addition, scattered neuroendocrine cells positive for chromogranin A and/or synaptophysin were seen in all cases. In 3 cases (two cases in the gallbladder and one case in the esophagus), areas of intestinal metaplasia with CK20, CDX2, and MUC2 positivity were found. Focal low-grade dysplasia was found in five cases (21.7%), and diffuse high-grade dysplasia was seen in one adenoma (4.4%), i.e., 6 of 23 PGAs (26.1%) showed dysplastic features. In one esophageal case, an invasive adenocarcinoma was diagnosed. Scattered p53 positive cells were found in all cases. Their number was higher in lesions with low-grade dysplasia and it was substantially increased in adenoma with high-grade dysplasia and in adenocarcinoma. Our molecular genetic results indicate that pyloric gland adenoma’s neoplastic nature is associated with p53 accumulation, mutations in oncogenes GNAS, KRAS, CTTNB1 and tumor suppressor genes SMAD4, and TP53. Pyloric gland adenoma can evolve into dysplasia and adenocarcinoma.
Keywords:
pyloric gland adenoma – gastric mucins – gastric metaplasia and heterotopia – dysplasia – adenocarcinoma – mutation
Pyloric gland adenoma (PGA) is a recently described lesion of the digestive system. It occurs in the esophagus, stomach, duodenum, pancreatobiliary system including the gallbladder, and in the rectum (1-12). PGA occurs predominantly in old patients, more frequently in women than in men. Endoscopically, it appears usually as a small nodular or papillary lesion, but rarely, it can reach a size of several centimeters (3). Histologically, the tumor is composed of closely packed tubular glands resembling pyloric glands, with a superficial layer of apical gastric epithelium. The main importance of diagnosis of PGA is in an uncertain behavior of this lesion, with observed malignant transformation in 12-30% of cases (2,3,5,8,10,11). It is suggested that PGA develops in the mucosa with pyloric metaplasia due to chronic irritation or in the gastric heterotopia of the gastrointestinal tract (3-6,8,10-13). We would like to present our series of 23 PGAs examined histologically, immunohistochemically, and with molecular genetic methods. We have focused also on the occurrence of intestinal metaplasia, dysplasia and malignant transformation. Because it had been suggested that PGA could arise in gastric heterotopias (5), we searched in a control group of duodenal and esophageal gastric heterotopias for MUC6-positive pyloric gland differentiation.
PATIENTS AND METHODS
Our study included endoscopic biopsies and cholecystectomies from 23 patients with PGA (11 men and 12 female, mean age 74.5 years, age range 52 – 87 years). The majority of PGAs involved the duodenum (10 cases) and stomach (7 cases). The remaining lesions were removed from the distal esophagus (3 cases), gallbladder (2 cases) and choledochus (one case). In addition, 16 gastric heterotopias of the duodenum (15 cases) and esophagus (one case) were examined for expression of gastric mucins.
All biopsy specimens were processed routinely and stained with hematoxylin and eosin, AMP (alcian blue at pH 2.5, periodic acid – Schiff reagent). In addition, gastric and esophageal biopsies were stained with silver impregnation for Helicobacter pylori (HP). Immunohistochemistry was performed using the standard avidin-biotin complex peroxidase technique, and it included the following antibodies: MUC5AC (clone MRQ-19, Cell Marque), MUC6 (clone MRQ-20, Cell Marque), CK20 (clone Ks20.8, Dako), MUC2 (clone MRQ-18, Cell Marque), CDX2 (clone EPR2764Y, Cell Marque), p53 (clone D07, Ventana), chromogranin A (clone DAK-A3, Dako), synaptophysin (clone SP11, Ventana), and Ki-67 (clone 30-9, Ventana). Cell proliferation (MIB1 index) was examined in 10 high power fields. The slides were examined by four pathologists (AC, TW, PM, and MZ).
Ten cases were selected to undergo molecular-genetic analyses. Where sufficient non-tumor material was available the samples were macrodissected into two parts separating the tumor and non-tumor components. DNA was extracted using the QIAsymphony DNA Mini Kit (QIAGEN, Hilden, Germany) on an automated extraction system (QIAsymphony SP, QIAGEN) according to the manufacturer’s supplementary protocol for FFPE samples (purification of genomic DNA from FFPE tissue using the QIAamp DNA FFPE Tissue Kit and deparaffinization solution). Concentration and purity of isolated DNA were measured using the NanoDrop ND-1000 (NanoDrop Technologies, Inc., Wilmington, DE, USA). DNA integrity was examined by amplification of control genes in multiplex PCR, producing fragments from 100 to 600 bp (14). Only cases with DNA integrity equal to or higher than 400 bp were tested using an array comparative genomic hybridization (aCGH) assay. Cases with DNA integrity equal or higher than 200 bp were selected for mutational analysis of hotspot mutations using next gene sequencing. Array comparative genomic hybridization was performed using CytoChip Focus Constitutional (BlueGnome Ltd., Cambridge, UK) as described elsewhere (15). Mutation analysis of hotspot regions of 50 oncogenes and tumor suppressor genes were analyzed using Ion AmpliSeq Cancer Hotspot Panel v2 (Life Technologies, part of Thermo Fisher Scientific, Waltham, MA USA) on an Ion Torrent Personal Genome Machine. Extracted DNA (10 ng) was amplified and adapters were ligated using an Ampliseq library preparation kit, sequencing beads were templated and enriched using a Template OT2 200 Kit. Sequencing was performed on a 316 chip with up to eight samples per chip using Sequencing kit 200 v2 (Life Technologies) according to the manufacturer’s protocols. Signal processing, mapping and quality control was performed with Torrent Suite v.4.2 (Life Technologies). Sequence variants were called using Ion Reporter v4.2 using AmpliSeq CHPv2 single sample workflow and default settings. Variants were subsequently filtered to include only exonic, non-synonymous variants with allele frequency higher than 5%. All filtered variants were annotated using HGVS nomenclature according to transcripts NM_001904.3, NM_033632.3, NM_080425.2, NM_033360.3, NM_005359.5, NM_001276760.1 of genes CTNNB1, FBXW7, GNAS, KRAS, SMAD4, and TP53 respectively.
RESULTS
PGAs in 16 of 23 cases (69.5 %) measured from 2 mm to 3 mm. In 7 cases the size was 5-10mm, two gallbladder lesions measured 10mm each. Histologically, PGAs showed typical zonal differentiation, with pyloric-like glands in the depth of adenoma and small superficial layer of tall columnar apical gastric epithelium (Fig. 1A). The narrow or cystically dilated tubules lying close together were lined by cuboidal to low columnar epithelial cells containing clear or pale eosinophilic cytoplasm. The basally located tumor cell nuclei tended to be round or oval, and they usually lacked prominent nucleoli. Paneth cells, oncocytic cells, and squamoid morules were not found. Immunohistochemically, PGAs expressed MUC6 (a pyloric gland mucin marker) in most tubules (Fig. 1B). The epithelium of the surface of the lesions expressed MUC5AC (a foveolar mucin marker) (Fig. 1C). In addition, focal MUC5AC positivity was also observed in the tubules. Neuroendocrine cells were found in all cases. They expressed chromogranin A and/or synaptophysin, and they were seen as scattered isolated cells or small group of cells. Two PGAs of gallbladder and one esophageal PGA showed focal intestinal metaplasia (Fig. 2A) with CK20 and CDX2 labeling. One PGA of the gallbladder and one esophageal PGA also showed MUC2 expression in the superficial and deep glandular epithelium (Figs. 2B and C). A majority of PGAs showed bland appearing cells with only scattered p53-positive nuclei and low proliferative activity (5 - 10 % of the cells were reactive for MIB-1). However, 5 of 23 lesions (21.7 %) contained foci of low-grade dysplasia (Fig. 3A) (two cases in the stomach, and one case in the esophagus, the ductus choledochus and gallbladder, respectively). In one esophageal PGA (i.e., 4.4 % of all cases), a diffuse high-grade dysplasia was found (Fig. 3B). Low-grade dysplasia showed more intense p53 expression than non-dysplastic PGAs (where there was only scattered and weak “wild-type“ immunopositivity), and increased cell proliferation with MIB1 index 20 - 35 % (Figs. 3C and D). In another esophageal PGA, a transformation to poorly differentiated adenocarcinoma was found (Fig. 3E). In high-grade dysplasia and adenocarcinoma, a production of gastric type mucins MUC6 and MUC5AC was still preserved, and the neoplastic cells showed diffuse p53 positivity and high cell proliferation with MIB1 index 80 % (Figs. 3F-H). Follow-up was not available for these two cases. Localizations of PGAs, the gender of the patients, and the occurrence of dysplasia/adenocarcinoma are summarized in Table 1.
Fig. 1. Pyloric gland adenoma. 
A: the tumor contains tubules of various size and columnar epithelium, B: strong MUC6 positivity is seen in the glands, and, focally, in the surface epithelium, C: diffuse MUC5AC expression in the surface epithelium and in adjacent tubules (A: hematoxylin and eosin, original magnification x40; B and C: ABC technique, original magnifications x40). Fig. 2. Pyloric gland adenoma with intestinal metaplasia. 
A: Focal intestinal metaplasia in the adenoma, B: CDX2 positivity, C: expression of MUC2 in goblet cells of intestinal metaplasia (A: hematoxylin and eosin, original magnification x100; B and C: ABC technique, original magnifications x40). Fig. 3. Pyloric gland adenoma with dysplasia. 
A: pyloric gland adenoma with low-grade dysplasia, B: pyloric gland adenoma with high-grade dysplasia, C: p53 in adenoma with low-grade dysplasia, D: MIB1 positivity in adenoma with low-grade dysplasia, E: poorly differentiated adenocarcinoma in the pyloric gland adenoma of the esophagus, F: MUC6 in adenoma with highgrade dysplasia, G: MUC5AC expression in superficial and glandular epithelium of the adenoma with highgrade dysplasia, H: p53 positivity in adenoma with high- grade dysplasia (A, B and E: hematoxylin and eosin, original magnifications x40, x40, and x100, espectively; C, D, F-H: ABC technique, original magnifications x40). 1. Pyloric gland adenomas. Localizations, gender, and occurrence of dysplasia. 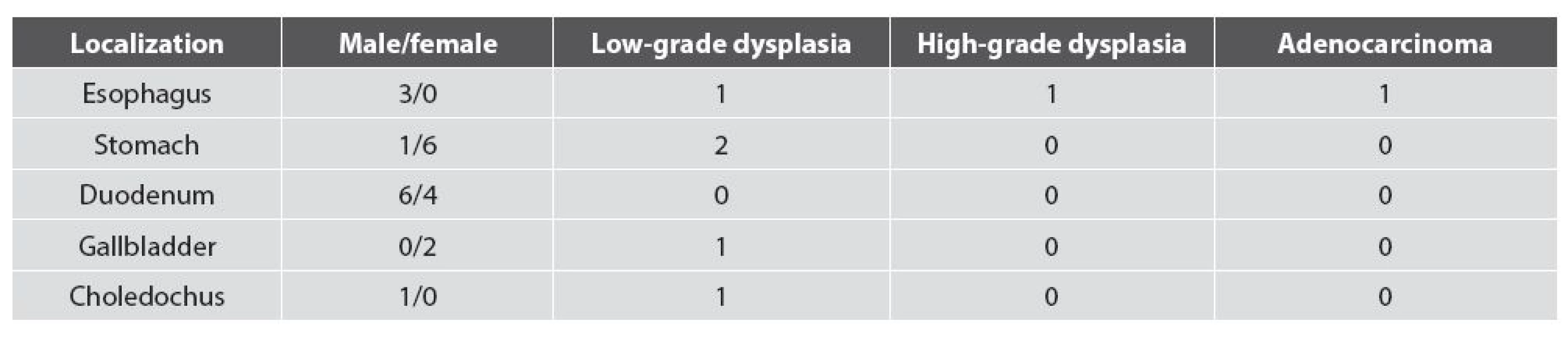
All gastric PGAs were found in the corporal mucosa with features of autoimmune gastritis. They occurred mostly in women (6 of 7 gastric cases). One esophageal PGA developed in columnar epithelium of the Barrett’s mucosa. In two additional cases, the mucosa adjacent to PGA was not present in the specimen. Silver impregnation for Helicobacter pylori was negative in all gastric and esophageal cases. In the duodenum, adjacent mucosa was normal in all cases (i.e., it lacked gastric heterotopia). For the differentiation between PGA and Brunner glands in small biopsy specimens, the use of MUC5AC was very helpful, because MUC5AC is positive in the superficial and glandular part of PGA whereas it is negative in Brunner glands. In choledochal PGA, an adjacent mucosa was not present in the specimen. In both gallbladder cases, the adjacent mucosa showed chronic inactive inflammation with extensive pyloric metaplasia and intestinal metaplasia. Both pyloric and intestinal metaplasia were seen in Rokitansky-Aschoff sinuses and in the superficial epithelium synchronously. However, pyloric metaplasia tended to occur in the basal parts of the sinuses. On the contrary, the intestinal metaplasia was more conspicuous in the superficial parts of the sinuses and in the superficial epithelium, respectively. In these areas, rare foci of gastric foveolar metaplasia were also found.
The control group of 16 gastric heterotopias included 15 duodenal cases (Fig. 4A) and one esophageal case. In all cases, MUC6 positive tubular glands were found (Fig. 4B), and MUC5AC expression was seen in superficial foveolar epithelium of the lesions (Fig. 4C).
Fig. 4. Control group of gastric heterotopias. 
A: a duodenal case of the gastric heterotopia, B: MUC6 positivity in the glands of the lesion, C: MUC5AC expression seen in the foveolar epithelium and adjacent glands (A: hematoxylin and eosin, original magnification x40; B and C: ABC technique, original magnifications x40). Fig. 5. aCGH chart depicting chromosomal aberrations in a case from esophageal PGA with high-grade dysplasia (x - chromosome number, y - log2 ratio of signal fluorescence of tested and reference sample). 
Molecular genetic results
Nine cases were analyzed by aCGH. One gastric PGA showed loss of the long arm of chromosome 5 and 6 (Fig. 6) and one esophageal case with high-grade dysplasia showed losses of chromosome 9, short arm of chromosome 17 and part of chromosome 21 (21q22). Normal (diploid) status of chromosomes was found in 7 cases.
Sequenced cases with average sequencing coverage over 500 x were included in the results. Of the 10 valid cases a total of 19 mutations were found (Tab. 2). The most frequently detected mutations were KRAS (7/10 cases) and GNAS (5/10 cases). In three cases, concurrent GNAS and KRAS mutations were detected. Two cases with a mutation in the beta-catenin gene (CTNNB1) were found. Both of these cases were wild type for KRAS and GNAS. Three SMAD4 mutations were detected, in each case coupled with another mutation (KRAS, CTNNB1, or FBXW7).
2. Molecular genetic results of array CGH and mutation analysis. 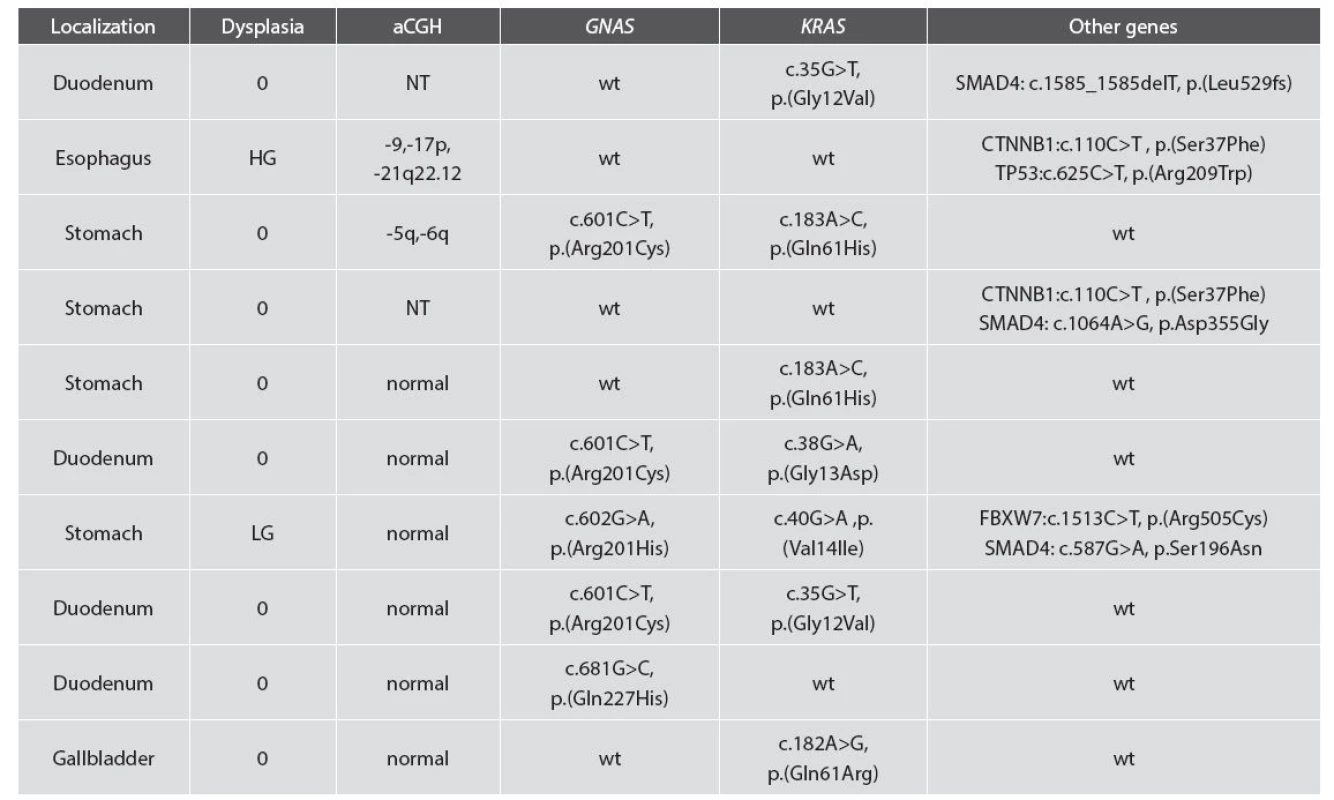
0: no dysplasia; LG: low-grade dysplasia; HG: high-grade dysplasia; wt: wild type. DISCUSSION
Pyloric gland adenoma was first described in the stomach by Borchard et al. (16) and simultaneously by Watanabe in the WHO classification of gastric tumors in 1990 (17). Similar adenomas were subsequently observed in Barrett´s esophagus (8,12), in gastric heterotopias or metaplasias of the gastrointestinal tract (5,9,11-13), and in pyloric metaplasias of the pancreatobiliary ducts and the gallbladder (1-6,10). PGA display an organoid growth pattern similar to that of a normal gastric mucosa with bidirectional differentiation, i.e., pyloric like glands are seen in the depth of the adenoma, and apical gastric-type epithelium is present in a superficial part of the lesion (8,12). The proportion of both of these components can vary (5). In some cases, the foveolar type differentiation prevails considerably, and then foveolar type cells are seen also in the depth of the adenoma, i.e., in the tubules (2,3,5). Such PGAs were originally, and not accurately, regarded as foveolar adenomas, despite the constant (although rudimentary) presence of pyloric-type glands in these lesions (3). The differentiation between pyloric - and foveolar-type epithelium is difficult without immunohistochemical stains. Although Chen et al. mention that PAS-positive apical mucin cap and elongated nuclei are typical for foveolar adenoma and do not occur in PGA (5), an immunohistochemical proof of MUC6 positivity is necessary for a reliable diagnosis of PGA (1,3,5,7,8,10,12,13,19). Currently, an already small number of MUC6 positive tubules in the lesion is regarded as diagnostic for PGA (5).
Squamoid morules which were found in PGAs in 20% of cases by Albores-Saavedra et al. (3) and even in 67% of cases by Adsay et al. (1) were not found in our series, neither in other studies (2,5,8,10,11).
Regarding adenomas of the gallbladder, recent WHO classification classifies as adenomas all exophytic glandular lesions which measure more than 1 cm (18). Thus, the difference between a focus of polypoid metaplasia and adenoma is based on an arbitrary threshold of 1cm. The types of adenomas are as follows: tubular, papillary, pyloric-gland type, foveolar type, biliary type and intestinal-type. The overlap between these subsets (hybrid patterns) is great, perhaps with the exception of the pyloric type. However, intestinal metaplasia was described in several PGAs of the gallbladder (1,3,6,10). We have found focal intestinal metaplasia in two cases of PGA of the gallbladder and in one esophageal PGA.
PGAs have long been considered benign lesions, but when the literature is analyzed it becomes clear that the larger lesions often do show dysplasia and carcinomatous transformation with changes in p53 expression. (1-3,5,8,11). Therefore, special care should be taken if a PGA is identified. Chen et al. (5) found in the series of 48 PGAs dysplastic features in 63.4 % of the lesions, and 51.2 % of them were of high-grade. In the series of Vieth et al. (11), even 30 % of cases showed focal transition to adenocarcinoma. It seems that the occurrence of dysplasia may be increased with the size of the lesion, at least in the pancreatobiliary PGAs. Adsay et al. (1) mention that gallbladder PGAs with size above 1cm had at least focal high-grade dysplasia in 88 % of cases, and carcinoma in 18% of cases, respectively. In our series, low-grade dysplasia was seen in 5 of 23 cases (21.7%), and included gastric, esophageal, choledochal and gallbladder PGAs. Dysplasia was found usually in the superficial epithelium of the lesions. It showed p53 positivity and increased cell proliferation (MIB1 index 20 - 40 %). In one of our esophageal PGAs, dysplasia was diffuse and of a high grade, and in another esophageal case a poorly differentiated adenocarcinoma was found in PGA. In comparison with previous studies, the frequency of dysplasia in our series was lower. We think that it can be well explained by the smaller size of our lesions (69.5 % of them measured 2 - 3 mm only).
PGAs arise from pyloric metaplasia of the gastrointestinal mucosa. In the stomach, it mostly occurs in a background of autoimmune gastritis (5). In our series, all gastric cases occurred in the setting of this gastritis. In contrast with the series of Vieth et al. (11), we were not capable of finding a single case of PGA in the gastric antrum. Esophageal PGAs were described in metaplastic specialized columnar epithelium with pyloric-gland type cells caused by reflux esophagitis, in Barrett’s esophagus and ectopic gastric fundic-type heterotopia, respectively (9). Our three esophageal PGAs were found in the distal esophagus, and their genesis was probably related to the reflux inflammation.
In our series, like in the series of Chen et al. (5), the most frequent localization of PGA was the duodenum (10 of our 23 cases). It was assumed that this high frequency is related to a high incidence of the duodenal gastric heterotopias which is up to 1.9 % of the population undergoing endoscopy (20). However, we have not found gastric heterotopia adjacent to PGA in any of our cases. Similarly, Chen et al. (5) found gastric heterotopia adjacent to PGA in only a single case. Another interesting aspect of the duodenal PGA is its diagnostic differentiation from Brunner glands which contain MUC6 positive pyloric-like cells, like PGA. We found immunostain for MUC5AC very useful in this setting, because PGA is usually MUC5AC positive in contrast with MUC5AC negative Brunner glands (5).
MUC6 positive cells were commonly found in heterotopic corporal gastric mucosa by Kushima et al. (21). We have verified this observation in our control series of such heterotopias. Thus, the gastric heterotopias contain cells with pyloric-like feature. For this reason, Kushima et al. (21) suggested the term “gastric heterotopia” instead of “ectopic gastric fundic-type mucosa”. This mixed antral-corporal phenotype of the heterotopic mucosa suggests that it may not represent a mere epithelial displacement during the fetal period of gastrointestinal system development, but may be reactive/metaplastic in nature (11,20).
In the pancreatobiliary tract, the gastric pyloric metaplasia of the ductal epithelium is associated with chronic inflammation and represents a common step in the pathogenesis of PGA. In the pancreas, PGA originates usually in the main duct (2,3,10). It is either isolated PGA (so-called type A) or it is associated with gastric type intraductal papillary mucinous neoplasia (so-called type B) (1,6). In contrast with intraductal papillary mucinous neoplasia which diffusely involves the entire circumference of the duct, PGA grows into the lumen as a polyp. In the gallbladder, our cases of PGA showed extensive pyloric and intestinal metaplasia in the adjacent mucosa. This finding further supports the assumption that PGA arises in pyloric metaplasia and that it can be associated with intestinal metaplasia.
Our genetic findings indicate that PGA represents a true neoplasia. Copy number aberrations or mutations were found in all studied cases, suggesting the potential of these adenomas to become malignant. In two cases (esophageal PGA with high-grade dysplasia and gastric PGA without dysplasia), copy number variations were found. In the case shown in Fig. 6, apart from a distinct loss of chromosomes 5p and 6p, several low-percentage changes are clearly visible in the background. This may be due to different percentages of mutated cells in the tumor sample suggesting intra-tumor heterogeneity and gradual oncogenesis. The second approach of mutational analysis of 50 genes known to be involved in oncogenesis revealed several expected findings as well as some novel ones. The high frequency of both KRAS and GNAS mutations and even the concurrent presence of these mutations in PGAs was already described by Matsubara et al. in 2013 (21). The higher frequency of KRAS mutations detected in our study than in Matsubara’s study can be attributed to analyzing all hotspots of KRAS. Our results further support former observations that GNAS and KRAS mutations are characteristic feature of PGAs. In addition we found two novel mutations (c.1585_1585delT and c.587G>A) and one known (c.1064A>G) SMAD4 mutations. To the best of our knowledge, this is the first time any mutations in SMAD4 were found in PGA. The tumor suppressor gene SMAD4 belongs in the Smad family, which mediates the TGF-beta signaling pathway suppressing epithelial cell growth. Somatic mutations of SMAD4 were described mostly in pancreatic and colorectal cancer, however they were also detected in gastric carcinoma (22). Loss of DPC4 (=SMAD4) expression was described in pyloric type of intraductal tubular adenoma of the pancreas (2). Interestingly, germline mutations of SMAD4 are causing familial juvenile polyposis, an autosomal dominant disease which is predisposed to gastrointestinal polyps and cancer. The fact that a TP53 mutation was detected in a case with strong p53 positivity is in correlation with the immunohistochemical result. The other strongly p53 positive case was not tested. Recent work showed frequent somatic beta-catenin mutations in duodenal gastric heterotopia, in some patients with the same mutation pattern in coexisting fundic gland polyps (23). Interestingly, we found beta-catenin mutation in two PGA cases: one esophageal, one gastric and none of the four duodenal cases examined. Whether there is a link between duodenal beta-catenin mutated duodenal gastric heterotopia and PGA, remains to be investigated.
In sum, our study demonstrated morphological, immunohistochemical and molecular genetic findings in PGAs. The most frequent localization of the adenoma was in the duodenum. The lesions showed differentiation toward pyloric gland-like tubules and superficial foveolar gastric cells, and sometimes even toward intestinal type epithelium. These various types of differentiation could be a sign of genetic instability of the epithelium, which can explain the relatively frequent finding of dysplasia or even carcinoma in PGA. The lesions often show p53 reactivity and increased cell proliferation. Molecular genetic analysis showed copy number aberrations and mutations in oncogenes GNAS, KRAS, CTTNB1 and tumor suppressor genes SMAD4, and TP53. Altogether, these findings support the opinion that PGA represents a true neoplasm.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
A. Chlumská, MD, CSc.
Biopticka lab.
Mikulasske nam. 4, 32600 Pilsen, Czech Republic
e-mail: chlumska@medima.cz
tel.: +420-737-220-403
Sources
1. Adsay V, Jang KT, Roa JC, et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥1.0 cm). Clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol 2012; 36(9): 1279-1301.
2. Albores-Saavedra J, Sheahan K, O´Riain C, Shukla D. Intraductal tubular adenoma, pyloric type, of the pancreas. Additional observations on a new type of pancreatic neoplasm. Am J Surg Pathol 2004; 28(2): 233-238.
3. Albores-Saavedra J, Chablé-Montero F, González-Romo MA, Ramírez-Jaramillo M, Henson DE. Adenomas of the gallblader. Morphologic features, expression of gastric and intestinal mucins, and incidence of high-grade dysplasia/carcinoma in situ and invasive carcinoma. Hum Pathol 2012; 43(9): 1506-1513.
4. Amaris J. Intraductal mucinous papillary tumor and pyloric gland adenoma of the pancreas. Gastrointest Endosc 2002; 56(3): 441-444.
5. Chen ZM, Scudiere JR, Abraham SC, Montgomery E. Pyloric gland adenoma. An entity distinct from gastric foveolar type adenoma. Am J Surg Pathol 2009; 33(2): 186-193.
6. Chetty R, Serra S. Intraductal tubular adenoma (pyloric gland-type) of the pancreas: a reappraisal and possible relationship with gastric-type intraductal papillary mucinous neoplasm. Histopathology 2009; 55(3): 270-276.
7. Chlumská A, Mukešnabl P, Dušek M, Zámečník M. Adenom pylorických žlazek – benigní nádor s nejistým biologickým chováním. Kongresové noviny, No 1, p.3, Meeting of Czech and Slovak Gastroenterologist, Nov 7, 2013, Karlovy Vary, Czech Republic.
8. Kushima R, Vieth M, Mukaisho K, et al. Pyloric gland adenoma arising in Barrett´s esophagus with mucin immunohistochemical and molecular cytogenetic evaluation. Virchows Arch 2005; 446(5): 537-541.
9. Michal M, Čuřík R, Matler K, Beneš Z. Regarding the paper by Vieth et al. Virchows Arch 442/4 : 317-321. Virchows Arch 2003; 443(4): 589-590.
10. Nakayama Y, Inoue H, Hamada Y, et al. Intraductal tubular adenoma of the pancreas, pyloric gland type. Am J Surg Pathol 2005; 29(5): 607-616.
11. Vieth M, Kushima R, Borchard F, Stolte M. Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Arch 2003; 442(4): 317-321.
12. Vieth M, Kushima R, de Jonge JPA, Borchard F, Oellig F, Stolte M. Adenoma with gastric differentiation (so-called pyloric gland adenoma) in a heterotopic gastric corpus mucosa in the rectum. Virchows Arch 2005; 446(5): 542-545.
13. Vieth M, Vogel C, Kushima R, Borchard F, Stolte M. Pyloric gland adenoma- - how to diagnose? Cesk Patol 2006; 42(1): 4–7.
14. van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and Tcell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003; 17(12): 2257–2317.
15. Sperga M, Martinek P, Vanecek T, et al. Chromophobe renal cell carcinoma--chromosomal aberration variability and its relation to Paner grading system: an array CGH and FISH analysis of 37 cases. Virchows Arch 2013; 463(4): 563-573.
16. Borchard F, Ghanei A, Koldovsky U, Hengels KJ, Buckmann FW. Gastrale Differenzierung in Adenomen der Magenschleihaut. Immunohistochemische und elektronenmikroskopische Untersuchungen. Verh Dtsch Ges Pathol 1990; 74 : 528.
17. Watanabe H, Jass JR, Sobin LH, eds. Histological typing of oesophageal and gastric tumours. Berlin Heidelberg: Springer-Verlag; 1990.
18. Bosman FT., Carneiro F, Hruban RH, Thiese ND, eds. World Health Organisation: International Agency for Research on Cancer. WHO classification of tumours of the digestive system. (4th edn), Lyon: IARC Press; 2010.
19. Lee SE, Kang SY, Cho J, et al. Pyloric gland adenoma in Lynch Syndrome. Am J Surg Pathol 2014; 38(6): 784-792.
20. Conlon N, Logan E, Veerappan S, McKiernan S, O`Briain S. Duodenal gastric heterotopia: further evidence of an association with fundic gland polyps. Hum Pathol 2013; 44(4): 636-642.
21. Kushima R, Borchard F, Hattori T. A new aspect of gastric metaplasia in Crohn´s disease: Bidirectional (foveolar and pyloric) differentiation in so-called pyloric metaplasia in the ileum. Pathol Int 1997; 47(6): 416-419.
22. Matsubara A, Sekine S, Kushima R, et al. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol 2013; 229(4): 579-587.
23. Miyaki M, Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun 2003; 306(4): 799-804.
24. Nakagawa M, Kitazawa R, Kondo T, et al. Duodenal gastric heterotopia, sporadic or fundic gland polyp-associated, frequently carries β-catenin mutation. Virchows Arch 2014; 465(3): 253–256.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2015 Issue 3-
All articles in this issue
- Tubulointerstitial rejection of renal allografts
- Antibody-mediated rejection of renal allograft and the update Banff classification 2013
- Infections after kidney transplantation
- Neuroendocrine tumor of the breast - metastasis or primary breast carcinoma? Case report
- Melanocytic matricoma - a case report
- WHAT IS YOUR DIAGNOSIS? Answer
- What si your diagnosis?
- Shadow cell differentiation in endometrioid carcinomas of the uterus. Its frequent occurrence and beta-catenin expression
- Pyloric gland adenoma: a histologic, immunohistochemical and molecular genetic study of 23 cases
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Pyloric gland adenoma: a histologic, immunohistochemical and molecular genetic study of 23 cases
- Infections after kidney transplantation
- Neuroendocrine tumor of the breast - metastasis or primary breast carcinoma? Case report
- Antibody-mediated rejection of renal allograft and the update Banff classification 2013
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

