-
Medical journals
- Career
Nephroprotective effect of N-acetylglucosamine in rats with acute kidney injury
Authors: Sergii K. Shebeko; Igor A. Zupanets; Olha O. Tarasenko
Published in: Čes. slov. Farm., 2019; 68, 173-179
Category: Original Article
Overview
The article presents the results of the study of the nephroprotective effect of N-acetylglucosamine (NAG) under the development of experimental acute kidney injury (AKI). The study was conducted on a model of acute glycerol nephrosis in rats. NAG was studied at a dose of 50 mg/kg at daily parenteral administration during 1 week compared to quercetin, which was administered intraperitoneally at a dose of 34 mg/kg. The efficiency of the drugs was assessed by the functional state of animals, the renal excretory function and the nitrogen metabolism indices. The NAG effect on rats with AKI caused a reduction of the mortality rate, an increase in diuresis, a reduction of proteinuria, an increase in creatinine and urea excretion, which indicates the normalization of the renal excretory function and nitrogen metabolism. At the same time, NAG has statistically significantly exceeded the effect of quercetin in the majority of indices and, therefore, the level of efficiency. Thus, NAG is an efficient agent for AKI treatment, which can be used at parenteral route of administration.
Keywords:
N-acetylglucosamine – nephroprotective effect – experimental acute kidney injury
Introduction
Treatment of acute kidney injury (AKI) is an important problem of modern medical and pharmaceutical practice. AKI is a common complication of not only renal pathology, but also of different diseases, and it also can be a result of drug therapy and other palliative or surgical treatment methods1). According to the data of world statistics, the prevalence rate of AKI is 5–10% of all indoor patients2). Much often AKI occurs in critically ill patients reaching the prevalence rate of up to 67%, herewith, in 5% a severe AKI develops, which requires renal replacement therapy3). Epidemiological studies demonstrate that AKI gradually spreads, since during over last 25 years its morbidity has increased almost by 20 times2). The greatest danger at AKI is mortality, which is up to 15% in the overall population of patients with AKI and reaches 80% in patients in critical state3). Therefore, optimization of AKI treatment and widening of inventory of efficient nephroprotective drugs are an important task of modern pharmaceutical science.
As the means for the solution of this problem, the drugs on the basis of natural membrane protectors can be implemented, whose properties include a nephroprotective effect with different mechanisms of action. With this purpose the study of different amino sugar glucosamine (GA) derivatives is prospective, since GA is a natural human metabolite and is practically safe for the organism4, 5). GA as a part of glycosaminoglycans and glycoproteins is comprised in the structures of biological membranes, in particular, in glomerular basement membrane, which stipulates its nephroprotective properties6). In the earlier experimental research we have proven the efficacy thereof upon the conditions of oral administration at glomerulonephritis7) and membranous nephropathy8).
However, GA nephroprotective action is slow, which is the drawback thereof. It implements its pharmacological effects through the biologically active form – N-acetylglucosamine (NAG) – and only in this form it is included in the structures of the damaged membranes, i.e. only after acetylation9). At the same time, in the case of oral administration, GA is transformed to NAG in the liver with the output of not more than 44%, the rest of amino sugar participates in energy metabolism10).
NAG is an active metabolite of GA, therefore, it potentially causes more expressed nephroprotective effect by the direct mechanism of action. Herewith, NAG has obvious advantages upon the condition of parenteral administration, since this route of administration allows neutralizing the influence of the effect of first-pass metabolism and providing the intake of the entire administered dose of NAG to blood circulatory system in the unchanged form. All this allows assuming high efficacy of this drug by nephroprotective action not only at latent course of kidney diseases, but also at acute injuries and exacerbations of chronic nephropathies.
In view of the above-mentioned, the purpose of this work is the study of the efficiency of N-acetylglucosamine at parenteral administration under development of AKI in rats.
Experimental part
Materials
NAG in the form of a 6% solution for injections was obtained from PJSC SIC Borschahivskiy CPP (Ukraine) as a pilot series. Quercetin was used in the form of the commercial drug Corvitin® (PJSC SIC Borschahivskiy CPP, Ukraine), which is a lyophilized powder for injections. Highly purified glycerol from Sigma-Aldrich (USA) was used to induce AKI. The biochemical assays were performed using the commercial kits Creatinine FS (cat. No. 117119910021), Urea FS (cat. No. 131019910021) and Total protein UC FS (cat. No. 102109910021) manufactured by DiaSys Diagnostic Systems GmbH (Germany).
Methods
Test object and its preparation
NAG was used as the object of research in the form of a 6% solution for injections. It was diluted immediately prior to use with a 0.9 % sodium chloride solution for injections to the concentration of 20 mg/ml.
Reference object and its preparation
Reference object was Corvitin®, which is an injectable dosage form of quercetin. This drug has a reliable nephroprotective effect at intraperitoneal (i.p.) administration in rats at a dose of 34 mg/kg, which is proven in experimental studies on different models of nephropathy11). Corvitin® was diluted immediately prior to use with a 0.9% sodium chloride solution for injections to the concentration of 20 mg/ml.
Experimental animals and grouping
Adult random-bred male albino rats with the body weight of 170–190 g were included in the present study. The rats were obtained from the vivarium of the Central Research Laboratory (National University of Pharmacy, Kharkiv, Ukraine) in the amount of 48 animals. The rats were housed under conventional laboratory conditions in standard polypropylene cages in a well-ventilated room at 25 ± 1 °C and a relative humidity 55 ± 5% with a regular 12 h light/12 h dark cycle12, 13). The animals received standard rat diet and water ad libitum.
All researches were conducted in accordance with the EU Directive 2010/63/EU on compliance with the laws, directives and administrative regulations of the EU countries on the protection of animals used for scientific purposes14). Study design was approved by the Bioethics Commission of the National University of Pharmacy (Protocol No. 1 of 20 January 2016).
All animals were randomly divided into 5 experimental groups as follows:
- group 1 – intact control (healthy animals receiving vehicle intramuscularly (i.m., n = 8)
- group 2 – control pathology (untreated animals receiving vehicle i.m., n = 10)
- group 3 – animals with AKI treated with NAG i.m. at 50 mg/kg (n = 10)
- group 4 – animals with AKI treated with NAG intravenously (i.v.) at 50 mg/kg (n = 10)
- group 5 – animals with AKI treated with Corvitin® (i.p.) at 34 mg/kg (n = 10)
Study design
On the first day of the experiment, AKI was induced in rats with an i.m. injection of 50% solution of glycerol (Sigma-Aldrich, USA) at a dose of 10 ml/kg into the muscles of the hind limbs, herewith, the animals were preliminary deprived of water for 24 hours15). After that animals received NAG i.m. and i.v. at a dose of 50 mg/kg, which corresponds to the effective dose of GA on the model of autoimmune glomerulonephritis7). Corvitin® was administered i.p. at a dose of 34 mg/kg, which corresponds to the ED50 by nephroprotective action11). All test samples were administered in the form of solutions for injections daily during a week. Animals of control groups received i.m. an equivalent dose of a 0.9% sodium chloride solution for injections. A week after the simulation of pathology in rats, the functional state of the kidneys was assessed. Further, they were taken out of the study with the purpose of obtainment of their blood and urine for biochemical assays.
Biological samples preparation and storage
At the end of the experiment, rats were sacrificed under anesthesia with ketamine/xylazine (75/10 mg/kg, i.p.)16). Blood samples were collected from the inferior vena cava and were centrifuged at 1500 g at +4 ºC for 10 min using a refrigerated centrifuge Eppendorf 5702R (Eppendorf, Germany). Urine samples were collected using individual metabolic cages and were centrifuged at 500 g for 10 min. The supernatants were separated and used for biochemical assays. All biological samples were frozen and stored at –80 °C.
Evaluation of functional state of kidneys
At the end of the study, the spontaneous daily diuresis and the volume of consumed fluid in animals were determined in individual metabolic cages, and then the relative diuresis was calculated. In the collected urine, the level of the protein was determined and its daily urine excretion was calculated17). The glomerular filtration rate (GFR) was evaluated by the clearance of endogenous creatinine, the tubular reabsorption (TR) and urea clearance (UC) were also calculated, using the standard formulas17–19):
- GFR = Ucr × V / Pcr [Eq. 1]
- TR = (1 – Pcr / Ucr) × 100% [Eq. 2]
- UC = Uur × V / Pur [Eq. 3]
where Ucr is the urine creatinine concentration,
V is the daily diuresis,
Рcr is the plasma creatinine concentration,
Uur is the urine urea concentration, and
Рur is the plasma urea concentration.
Evaluation of biochemical parameters
In order to evaluate the parameters of nitrogen metabolism in animals, biochemical assays were performed using commercial kits and an Express Plus automatic biochemical analyzer (Bayer Diagnostics, Germany). The creatinine content was determined in the blood and urine of animals by the reaction of Jaffe and the urea content was determined using the urease-glutamate dehydrogenase method. The urinary excretion of creatinine and urea was calculated as well. The concentration of urine protein was determined by a photometric test based on the reaction with pyrogallol red-molybdate complex17).
Statistical analysis
All obtained results were processed by descriptive statistics and presented as the mean ± standard error of the mean (M ± SE) excluding the survival rate. Statistical differences between groups were analyzed using one-way ANOVA following Scheffe post-hoc test20). The analysis of animal survival was performed according to the Kaplan-Meier method21), while the generalised Wilcoxon test was used to detect differences between groups. The utilized computer software included IBM SPSS Statistics v. 22 (IBM Сorp.) and MS Excel 2016 (Microsoft Corp.).The level of statistical significance was considered as p < 0.05.
Results
The results of the study have demonstrated that a week after simulation of the pathology, low survival rate was observed in untreated animals, which was reduced to 50% (Fig. 1). Rats were floppy, inactive, with edema and ascites, food intake was limited.
1. Kaplan-Meier survival curves in rats with AKI under the influence of NAG (n = 48)
* significant relative to the intact control group (p < 0.05)
# significant relative to the control pathology group (p < 0.05)
n – number of animals in the experiment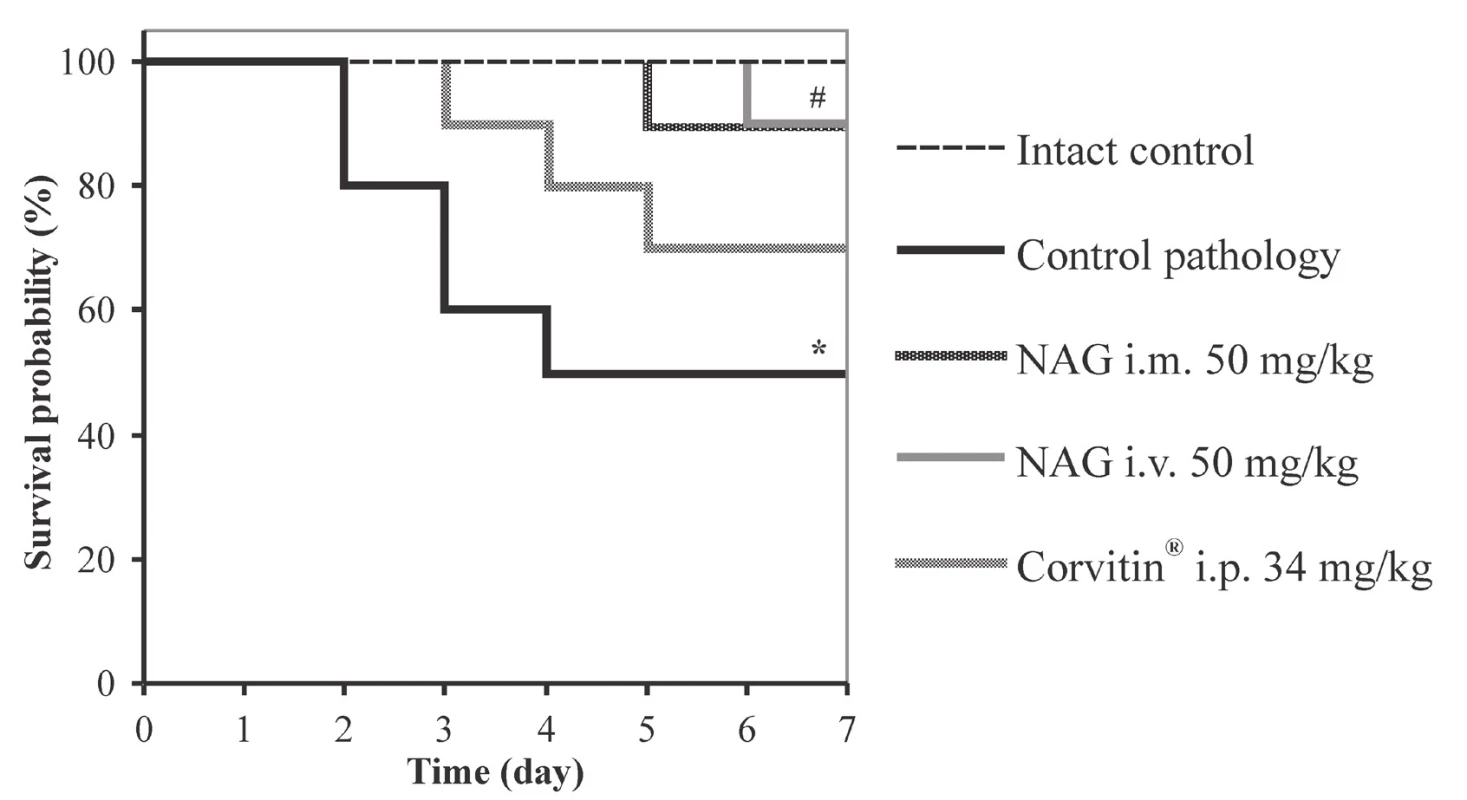
Herewith, the statistically significant reduction of spontaneous and relative diuresis by 1.9 and 1.5 times was observed, respectively, as compared to intact animals, as well of GFR by 4.4 times and TR to 96.39% (Table 1). Besides, expressed proteinuria was observed, which reached 41.3 mg/day (Fig. 2). All this evidences an impaired renal excretory function and the development of AKI.
1. Indices of excretory function of the kidneys under the effect of NAG on the background of AKI in rats (M ± SE, n = 48) 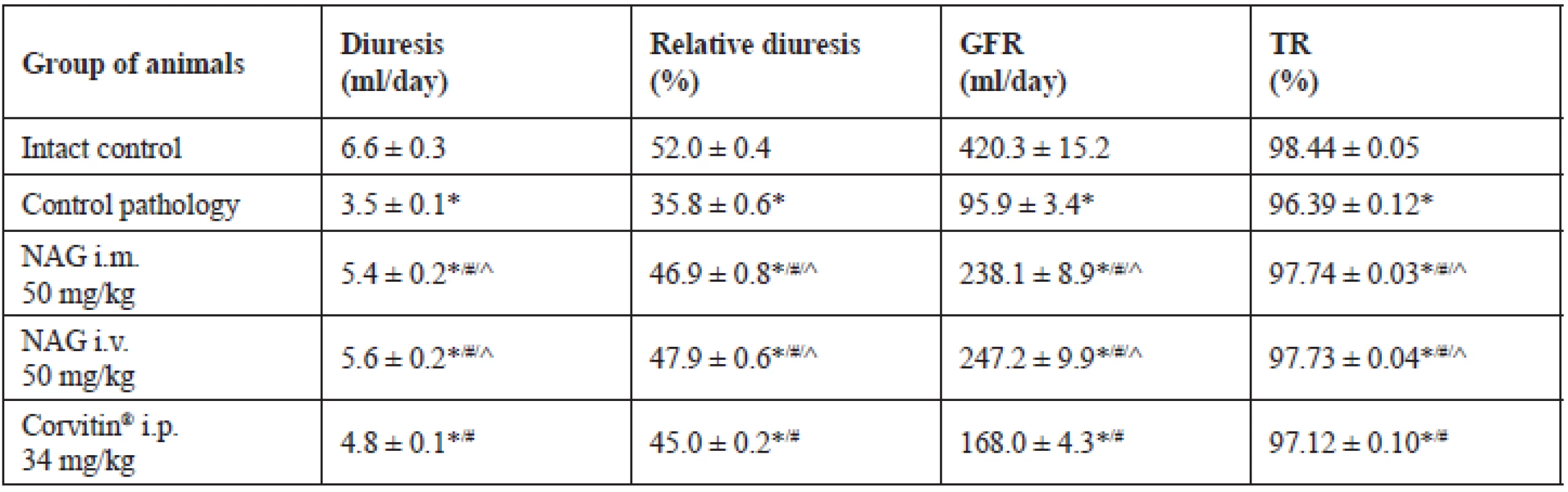
* significant relative to the intact control group (p < 0.05)
# significant relative to the control pathology group (p < 0.05)
^ significant relative to the animals treated with Corvitin® (p < 0.05)
n – number of animals in the experiment2. The influence of NAG on the proteinuria in rats with AKI (M ± SE, n = 48)
* significant relative to the intact control group (p < 0.05)
# significant relative to the control pathology group (p < 0.05)
^ significant relative to the animals treated with Corvitin® (p < 0.05)
n – number of animals in the experiment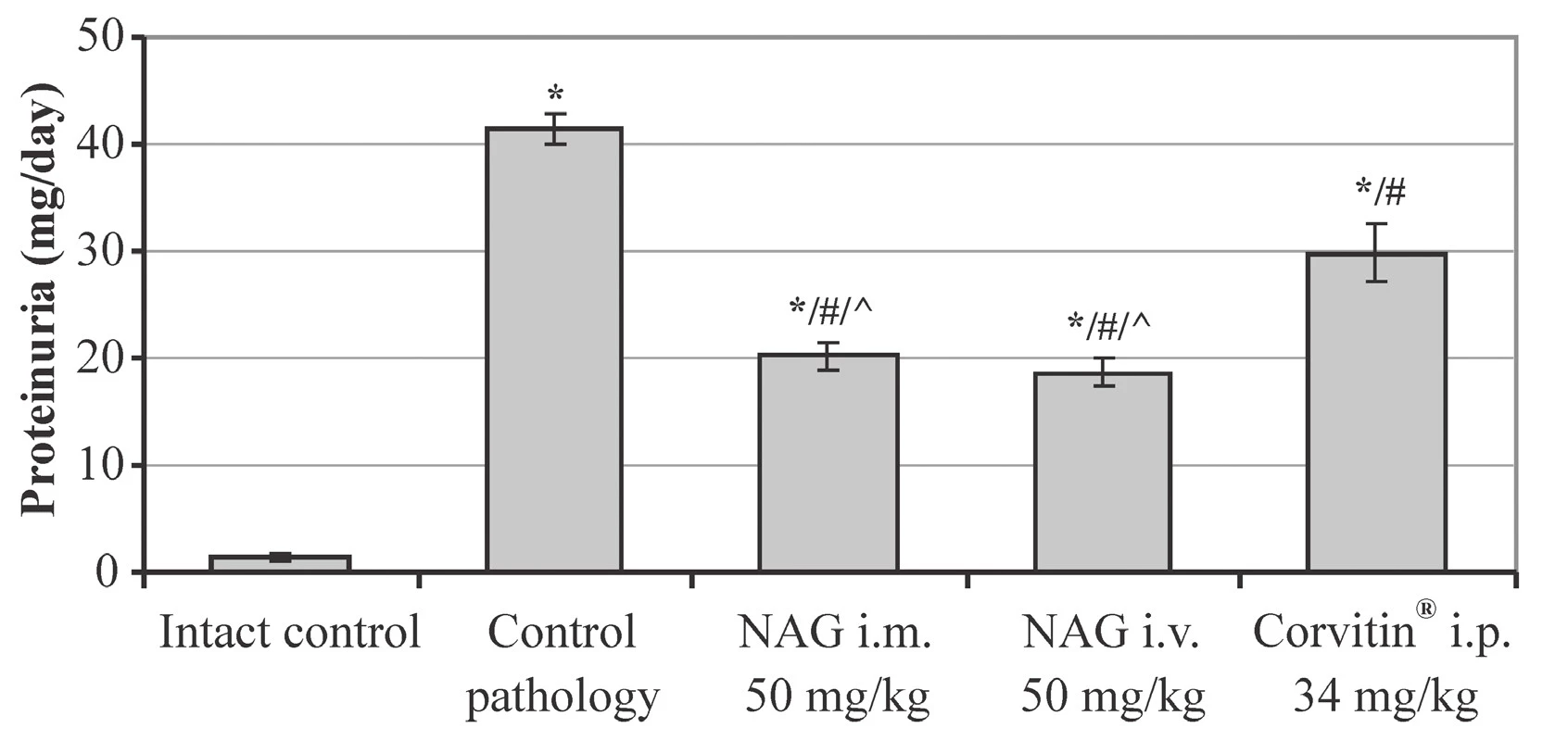
Due to this, a sharp reduction of excretion of nitrogen compounds occurred and azotemia developed in animals. The content of creatinine and urea in blood as compared to intact rats increased by 3.8 and 3.7 times, respectively. Herewith, urine excretion was reduced: of creatinine by 14 %, and of urea by 22% (Table 2).
2. The influence of NAG on nitrogen metabolism in rats on the background of AKI (M ± SE, n = 48) 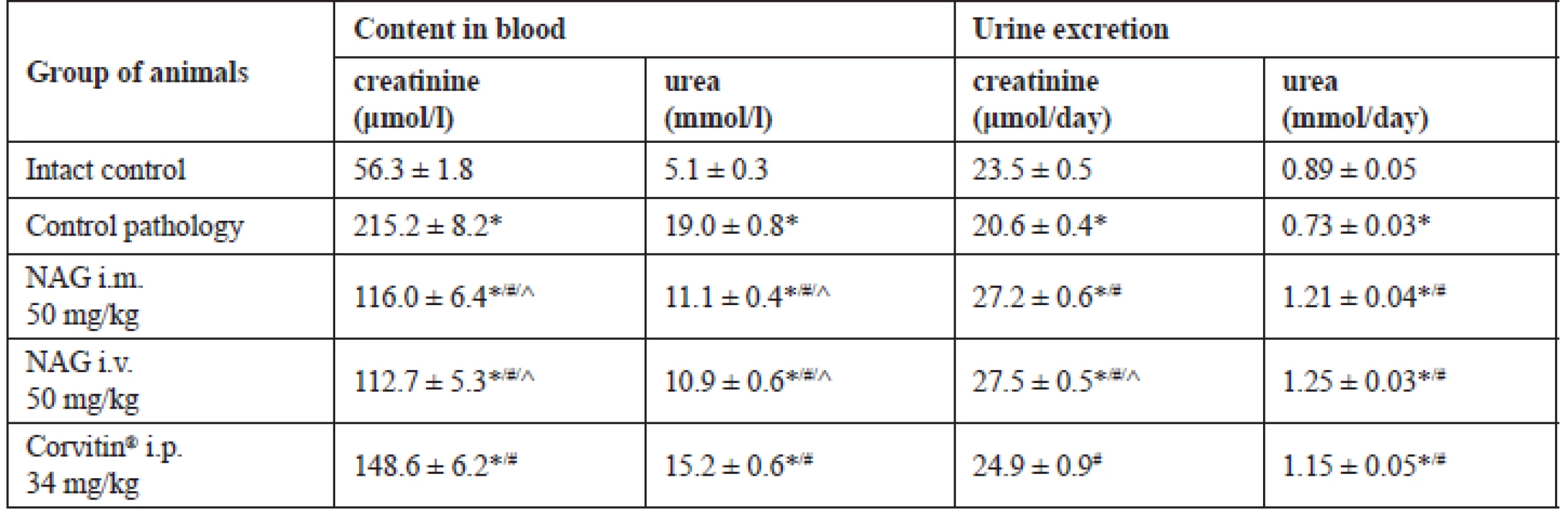
* significant relative to the intact control group (p < 0.05)
# significant relative to the control pathology group (p < 0.05)
^ significant relative to the animals treated with Corvitin® (p < 0.05)
n – number of animals in the experimentRespective changes were also observed in the UC index, which reflects the rate of blood purification from this substance. In the control pathology group this index reduced to 38.4 ml/day, which was reliably less by 4.6 times compared to intact rats (Fig. 3).
3. The influence of NAG on the urea clearance in rats with AKI (M ± SE, n = 48)
* significant relative to the intact control group (p < 0.05)
# significant relative to the control pathology group (p < 0.05)
^ significant relative to the animals treated with Corvitin® (p < 0.05) n – number of animals in the experiment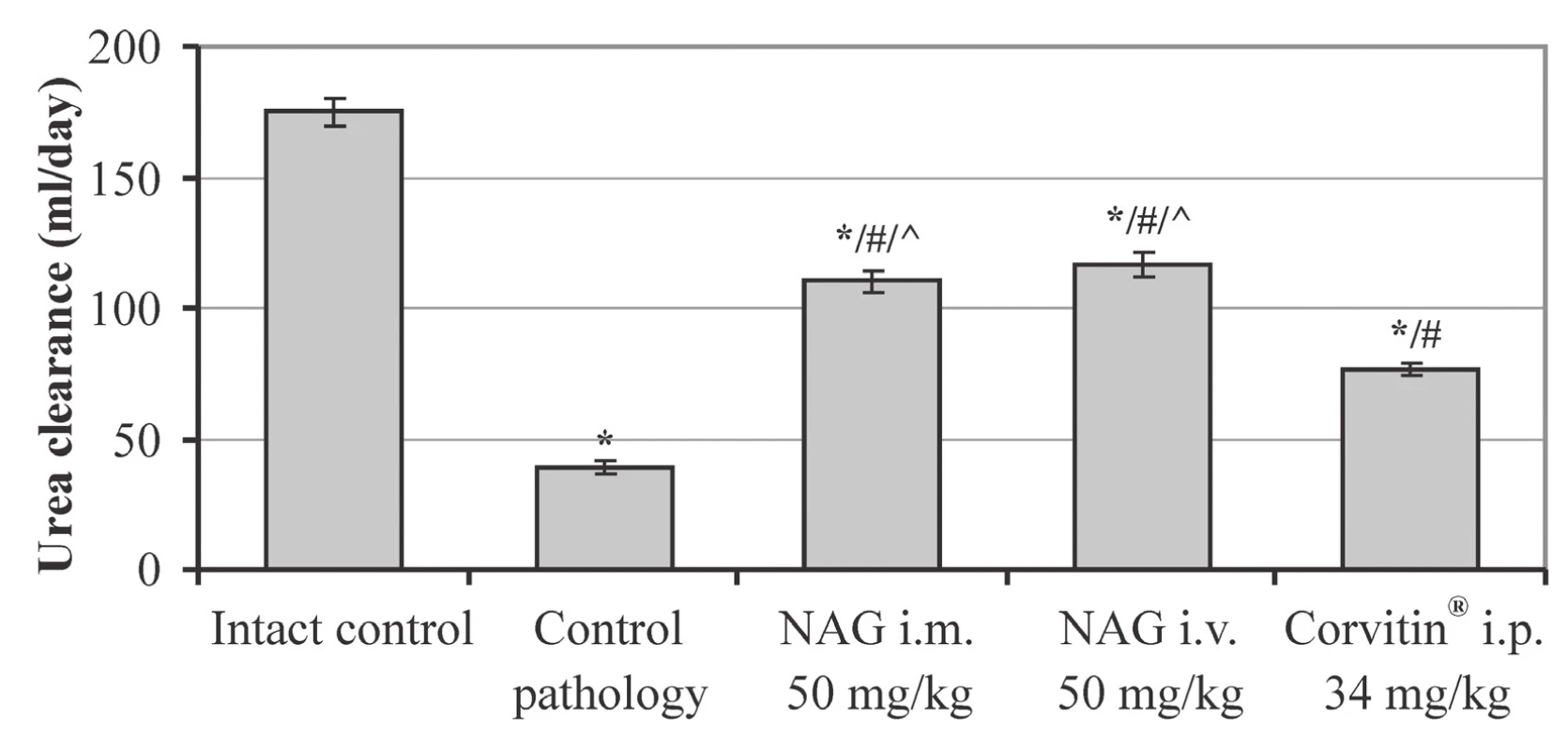
In contrast, when using NAG for treatment of animals, an expressed positive effect on the AKI course was observed. Under the effect of the amino sugar the functional state of rats normalized and the survival reliably increased to 90% (Fig. 1). The statistically significant improvement of renal excretory function was observed as compared to untreated animals. Herewith, spontaneous diuresis increased by 1.5 and 1.6 times at i.m. and i.v. administration, respectively, and relative diuresis by 1.3 times. GFR increased by 2.5 times at i.m. administration and by 2.6 times at i.v. administration, and also the TR index increased by 1.4% (Table 1). Furthermore, NAG facilitated statistically significant reduction of proteinuria by 2.0 at i.m. administration and by 2.2 times at i.v. administration (Fig. 2).
Positive changes under influence of NAG were also observed in nitrogen metabolism. Hexosamine for both routes of administration has statistically significantly increased urine excretion of creatinine relative to the control group by 1.3 times, and of urea by 1.7 times. Due to this, the content of creatinine in blood reduced by 1.9 times, and of urea by 1.7 times (Table 2). Besides, under influence of NAG the statistically significant increase in UC occurred: by 2.9 times at i.m. administration and by 3.0 times at i.v. administration (Fig. 3). The described picture evidences the improvement of the functional condition of the kidneys and normalization of the nitrogen metabolism in rats with AKI.
The reference drug Corvitin®, which is a parenteral form of quercetin, has demonstrated slightly lower level of efficacy. Under the influence thereof the survival rate in rats increased to 70%, which has no statistically significant distinctions from the untreated animals (Fig. 1). The spontaneous and relative diuresis statistically significantly increased by 1.4 and 1.3 times, respectively, the GFR index has increased by 1.8 times, and the TR by 0.76% (Table 1). Also, a statistically significant reduction of proteinuria was observed, by 1.4 times, as compared to the control pathology (Fig. 2).
Additionally, Corvitin® facilitated the creatinine excretion by 1.2 times and the urea excretion – by 1.6 times, and reduced their content in blood by 1.3–1.4 times (Table 2). At the same time, urea elimination from blood was improved, which was confirmed by the statistically significant increase in the UC by 2.0 times as compared to the untreated group (Fig. 3). All this evidences an improvement of the functional condition of the rat kidneys with AKI under the influence of Corvitin®, however, by the level of efficacy it statistically significantly conceded to NAG in the majority of studied indices.
Discussion
The analysis of the obtained data demonstrates that under the influence of i.m. administration of glycerol, in rats full-scale picture of AKI develops during a week. This is evidenced by stable oliguria, water retention in the organisms and a sharp reduction of the excretory function of the kidneys. Due to this, the excretion of nitrogenous substances is reduced, and azotemia and autointoxication develop in rats organisms. This causes worsening of their functional state and a high rate of mortality. The described situation is characteristic of an acute glycerol nephrosis and occurs under the influence of myoglobin, which is formed due to rhabdomyolysis and causes toxic-ischemic kidney injury3, 15). Therefore, this experimental model is stipulated by the toxic and ischemic mechanisms of the nephropathy development.
NAG has an expressed positive effect on AKI in rats with both routes of administration, i.m. and i.v., without statistical differences from each other. Studied hexosamine significantly improved the excretory function of the kidneys, reduced water retention, signs of edema, improved the excretion of nitrogen compounds with urine, and reduced the level of azotemia. As a result, the sings of intoxication in animals reduced, their general functional state was improved, and the survival rate increased to 90%.
Amino sugars from the group of GA derivatives were rarely studied as agents for the treatment of renal pathology. In previous researches, the nephroprotective properties of GA were studied in rats at oral administration in the conditions of different models of kidney injury7, 8). It was proved that GA integrates into the damaged structures of the kidney tissue and increases the content of endogenous hexosamines in it8). These results correlate with the data from other studies, which showed the efficacy of GA in the treatment of kidney fibrosis in mice22), contrast-induced acute kidney injury in rats23), and the efficacy of its conjugates in rats with renal ischemia/reperfusion injury24, 25). Research data of the effectiveness of NAG as a treatment of AKI have not been presented until now.
The comparator Corvitin®, which contains quercetin, showed a positive effect on AKI as expected. It significantly improved the excretory function of the kidneys, increased the excretion of nitrogen compounds, and reduced their blood content. These results are consistent with other researches, in which the nephroprotective properties of quercetin were studied and the prospects for its use in kidney diseases were proven11, 26–28).
It should be noted that NAG at both routes of administration has statistically significantly exceeded the action of Corvitin® on the level of efficacy in the most of indices. High efficiency of NAG is explained by the fact that it is an active metabolite of GA5) and causes a direct protective action on the damaged membrane structures of the kidney filter and intercellular substance by binding to the macromolecules thereof in the unchanged form6). At the same time, the influence of quercetin on the kidney tissue is indirect and develops as a result of antioxidant, antihypoxic, anti-inflammatory and other effects11, 29, 30). In this regard, the obtained data are very important for medicinal and pharmaceutical practice, since they discover a great prospect for the use of a new nephroprotective drug for the treatment of AKI – NAG in an injectable dosage form.
The nephroprotective effect of NAG is stipulated by the specific biological properties thereof. Being a part of glycosaminoglycans and glycoproteins, which cover the surface of the glomerular basement membrane, NAG not only performs the protective function, but also facilitates the creation of a negative charge on the membrane, which prevents the penetration of blood serum protein therethrough, and hence, the development of proteinuria6). Furthermore, the stabilization of the superficial charge of the basement membrane during nephropathy development causes normalization of hemodynamics in nephrons, which is inevitably impaired when the disease develops.
When comparing the efficacy of the amino sugar at different routes of administration, it turns out that it demonstrates the same level of activity without statistically significant differences both at i.m. and i.v. injection. However, at the same time, the i.m. route of administration is easier and more convenient when using the drug. Therefore, at further experimental research, as well as during the clinical trial, the i.m. route of administration for NAG should be considered as the most optimal one.
Thus, the obtained experimental data evidence that NAG in an injectable dosage form causes an expressed nephroprotective action in rats with AKI and is a prospective way of therapy of acute nephropathies, as well as of exacerbations of chronic renal pathology. It is expedient to use solution for injections of NAG upon conditions of i.m. administration, however, it can be also administered i.v. in accordance with the purpose of treatment.
Conclusion
The amino sugar NAG causes an expressed nephroprotective action at parenteral administration in the condition of AKI development in rats. Parenteral route of administration is an additional advantage for NAG, since it stipulates the intake of the entire administered dose of hexosamine in the active form to blood circulation. NAG statistically significantly exceeds the action of the reference drug Corvitin® (a quercetin parenteral form) by the degree of efficacy at AKI in rats. NAG in solution for injections is a prospective drug for AKI treatment, as well as of exacerbations of chronic renal pathology. It is expedient to further study the injectable dosage form of NAG at i.m. administration as a drug for therapy of renal pathology.
Conflict of interest: none.
Assoc. prof. Sergii K. Shebeko, PhD (∗)
I. A. Zupanets
O. O. Tarasenko
Department of Clinical Pharmacology and Clinical Pharmacy, National University of Pharmacy
27 Pushkinska str., 61057 Kharkiv, Ukraine
e-mail: shebeko.sk@gmail.com
Sources
1. Gilbert S. J., Weiner D. E., Bomback A. S., Perazella M. A., Tonelli M. National Kidney Foundation Primer on Kidney Diseases, 7th ed. Philadelphia: Elsevier 2018.
2. Lerma E., Sparks M., Topf J. Nephrology secrets, 4th еd. Philadelphia: Elsevier 2019.
3. Feehally J., Floege J., Johnson R. J., Tonelli M. Comprehensive clinical nephrology, 6th ed. Philadelphia: Elsevier 2019.
4. Baynes J. W., Dominiczar M. H. Medical biochemistry, 5th ed. Philadelphia: Elsevier 2019.
5. Lieberman M., Peet A. Marks’ basic medical biochemistry: A clinical approach, 5th еd. Philadelphia: Wolters Kluwer 2018.
6. Morita H., Yoshimura A., Kimata K. The role of heparan sulfate in the glomerular basement membrane. Kidney International 2008; 73, 247–248.
7. Shebeko S. K., Zupanets I. A. Study of the pharmacological properties of some glucosamine derivatives under conditions of development of experimental autoimmune glomerulonephritis. Klìnìčna farmacìâ 2006; 10(2), 31–35 (in Ukrainian).
8. Zupanets I. A., Shebeko S. K. The influence of experimental therapy on the dynamics of endogenous glucosamine content in laboratory animals with nephropathy. Eksp. Klin. Farmakol. 2006; 69(6), 40–42 (in Russian).
9. Chen J. K., Shen C. R., Liu C. L. N-Acetylglucosamine: production and applications. Mar. Drugs 2010; 8, 2493–2516.
10. Du Souich P. Absorption, distribution and mechanism of action of SYSADOAS. Pharmacology & Therapeutics 2014; 142(3), 362–374.
11. Shebeko S. K., Zupanets I. A., Popov O. S. Tarasenko O. O., Shalamay A. S. Effects of quercetin and its combinations on health. In: Watson R. R., Preedy V. R., Sherma Z. (eds.) Polyphenols: mechanisms of action in human health and disease, 2nd ed. London: Academic Press 2018; 373–394.
12. Guide for the care and use of laboratory animals, 8th ed. Washington: National Academies Press 2011.
13. Sharp P., Villano J. S. The laboratory rat, 2nd ed. Boca Raton: CRC Press 2013.
14. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union 2010; L276, 33–79.
15. Bao Y. W., Yuan Y., Chen J. H., Lin W. Q. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zoological Research 2018; 39(2), 72–86.
16. Flecknell P. A. Laboratory animal anesthesia, 4th ed. Oxford: Academic Press 2015.
17. Hart S. A., Hropot M., Greger R., Gögelein H., Bleich M. Activity on urinary tract. In: Vogel H. G. (ed.) Drug discovery and evaluation: Pharmacological Assays, 3rd ed. Berlin: Springer-Verlag 2008; 457–510.
18. Koeppen B. M., Stanton B. A. Renal physiology, 6th ed. Philadelphia: Elsevier 2019.
19. Speeckaert M., Delanghe J. Assessment of renal function. In: Turner N., Lameire N., Goldsmith D. J., Wineals C. G., Himmelfarb J., Remuzzi G. (eds.) Oxford textbook of clinical nephrology, 4th ed. Oxford: University Press 2016; 44–61.
20. Islam M. A., Al-Shiha A. Foundations of biostatistics. Singapore: Springer 2018.
21. Dudley W. N., Wickham R., Coombs N. An introduction to survival statistics: Kaplan-Meier analysis. J. Adv. Pract. Oncol. 2016; 7(1): 91–100.
22. Park J., Lee S. Y., Ooshima A., Yang K. M., Kang J. M., Kim Y. W., Kim S. J. Glucosamine hydrochloride exerts a protective effect against unilateral ureteral obstruction-induced renal fibrosis by attenuating TGF-β signaling. J. Mol. Med. 2013; 91(11), 1273–1284.
23. Hu J., Chen R., Jia P., Fang Y., Liu T., Song N., Xu X., Ji J., Ding X. Augmented O-GlcNAc signaling via glucosamine attenuates oxidative stress and apoptosis following contrast-induced acute kidney injury in rats. Free Radic. Biol. Med. 2017; 103, 121–132.
24. Wang X., Xiong M., Zeng Y., Sun X., Gong T., Zhang Z. Mechanistic studies of a novel mycophenolic acid-glucosamine conjugate that attenuates renal ischemia/reperfusion injury in rat. Mol. Pharm. 2014; 11, 3503–3514.
25. Fu Y., Lin Q., Gong T., Sun X., Zhang Z. R. Renal-targeting triptolide-glucosamine conjugate exhibits lower toxicity and superior efficacy in attenuation of ischemia/reperfusion renal injury in rats. Acta Pharmacol. Sin. 2016; 37, 1467–1480.
26. Yang H., Song Y., Liang Y. N., Li R. Quercetin treatment improves renal function and protects the kidney in a rat model of adenine-induced chronic kidney disease. Med. Sci. Monit. 2018; 24, 4760–4766.
27. Layal K., Perdhana I. S., Louisa M., Estuningtyas A., Soetikno V. The effects of quercetin on oxidative stress and fibrosis markers in chronic kidney disease rat model. Med. J. Indones. 2017; 26, 169–177.
28. Vargas F., Romecín P., García-Guillén A. I., Wangesteen R., Vargas-Tendero P., Paredes M. D., Atucha N. M., García-Estañ J. Flavonoids in kidney health and disease. Front. Physiol. 2018; 9, Article 394. https://www.frontiersin.org
29. Anand David A.V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacognosy Review 2016; 10(20), 84–89.
30. Li Y., Yao J., Han C., Yang J., Chaudhry M. T., Wang S., Liu H., Yin Y. Quercetin, inflammation and immunity. Nutrients 2016; 8(3), 167–181.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2019 Issue 4-
All articles in this issue
- Plant α-amylase inhibitors and their effect on the utilization of polysaccharides contained in the diet
- Theory and practice of pharmacopoeial control of quality of drugs and excipients X. Number of parallel determinations, processing of results and their use in the assessment of the content of active substances and excipients in the European Pharmacopoeia (Ph. Eur.)
- New approach for detoxification of patients dependent on benzodiazepines and Z-drugs for reduction of psychogenic complications
- Study of biocompatibility of peritoneal dialysis solutions measured as in vitro cells viability
- Nephroprotective effect of N-acetylglucosamine in rats with acute kidney injury
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- New approach for detoxification of patients dependent on benzodiazepines and Z-drugs for reduction of psychogenic complications
- Plant α-amylase inhibitors and their effect on the utilization of polysaccharides contained in the diet
- Nephroprotective effect of N-acetylglucosamine in rats with acute kidney injury
- Theory and practice of pharmacopoeial control of quality of drugs and excipients X. Number of parallel determinations, processing of results and their use in the assessment of the content of active substances and excipients in the European Pharmacopoeia (Ph. Eur.)
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

