-
Medical journals
- Career
TREATMENT OPTIONS FOR PREMACULAR AND SUB-INTERNAL LIMITING MEMBRANE HEMORRHAGE
Authors: M. Kováčová 1; B. Kousal 1; M. Meliška 1; M. Fichtl 1; J. Dušková 2; B. Kalvodová 1
Authors‘ workplace: Oční klinika, 1. lékařská fakulta, Univerzita Karlova a Všeobecná fakultní nemocnice v Praze 1; Ústav patologie, 1. lékařská fakulta, Univerzita Karlova a Všeobecná fakultní nemocnice v Praze 2
Published in: Čes. a slov. Oftal., 77, 2021, No. 6, p. 288-292
Category: Original Article
doi: https://doi.org/10.31348/2021/34Overview
Introduction: Premacular hemorrhage (PH) and sub-internal limiting membrane hemorrhage (sub-ILM-H) are among the causes of sudden deterioration of central visual acuity. Anatomical and functional outcomes of different therapeutic options were evaluated retrospectively.
Methods: The study included three eyes of three patients (2 females and 1 male). Location of the hemorrhage was determined by spectral domain optical coherence tomography. Subhyaloid premacular location of the hemorrhage was proven in one eye of each woman and sub-ILM location of the hemorrhage in one eye of the male. The baseline best corrected visual acuity (BCVA) was 0.63 in the eyes of the females and 0.16 in the eye of the male. Conservative treatment option was chosen in case of juxtafoveolar PH in the eye of the female patient on anticoagulant warfarin therapy. The female patient with PH secondary to proliferative diabetic retinopathy (PDR) underwent Nd: YAG laser hyaloidotomy. The male patient with unexplained cause of the sub - ILM-H underwent 25-Gauge vitrectomy with ILM peeling and subsequent ultrastructural morphometric and histopathological examination of the ILM.
Results: Both BCVA and retinal finding improvement were achieved in all patients. Final BCVA was 0.8 in the eye of the female patient with PDR and 1.0 in rest of the eyes of the other patients. No complications were recorded at follow-up visits. Histopathological and morphometric examination demonstrated variable ILM thickness (2.70 ±1.58 μm) and proved presence of fibroblasts and macrophages with hemosiderin deposits on the retinal side of ILM.
Conclusion: The choice of the treatment option of PH and sub-ILM-H depends on input parameters such as the initial BCVA, the extent and the location of the hemorrhage, as well as the overall health of the patient. Nd: YAG laser hyaloidotomy is an effective method for rapid recovery of visual functions. Surgical ILM peeling and aspiration of the underlying hemorrhage result in the removal of breakdown products of hemoglobin and minimization of the risk of secondary epiretinal membranes development.
Keywords:
histology – premacular hemorrhage – internal limiting membrane – ultrastructural morphometry – histopatology – Nd:YAG laser hyaloidotomy – 25-Gauge vitrectomy
INTRODUCTION
Premacular bleeding and bleeding under the inner limiting membrane (ILM) of the retina in the posterior pole of the eye is one of the causes of sudden deterioration in central visual acuity (CVA). We distinguish types according to the location of the bleeding - bleeding under the ILM, i.e., between the layer of retinal nerve fibres and the ILM, and subhyaloid bleeding, which is located between the ILM and the posterior vitreous membrane, or a combination of both. Biomicroscopically, subhyaloid hemorrhage is usually diffuse, with irregular edges, varying in saturation and changes shape with the patient's body position. The shape of bleeding under the ILM does not change with the patient's position and is well demarcated with cellophane glosses and has the shape of a dome with a double ring of ILM and a posterior vitreous membrane. The main causes of premacular bleeding and bleeding under the ILM include the Valsalva manoeuvre (sudden increase in intrathoracic and central venous pressure), subarachnoid haemorrhage, trauma, hematopoietic or prothrombotic conditions, hypertensive disease or ocular complications of diabetes mellitus.
MATERIAL AND METHODS
There were three eyes of three patients (2 women and 1 man) who were 60, 50 and 51 years of age respectively. Detailed eye examination included determination of the best corrected central visual acuity using the Snellen or Early Treatment of Diabetic Retinopathy Study (ETDRS) optotypes, expressed in decimal values. The fundus finding upon biomicroscopic examination in mydriasis was photographically documented (FF 450 plus IR and Visucam 200, Carl Zeiss Meditec AG, Jena, Germany). Individual macular layers were imaged using spectral domain optical coherence tomography (SD-OCT) using Spectralis (Heidelberg Engineering GmbH) and Spectral OCT / SLO (OTI Ophthalmic Technologies Inc., Toronto, Canada). In one patient, material from the vitreoretinal interface was sent for ultrastructural morphometric examination. Semi-thin and ultrathin sections were made and photographed in 40 examination locations (NIS-Elements, Laboratory Imaging, Prague, Czech Republic).
Patient No. 1, a 60-year-old woman, was examined for 2 weeks of worsened vision in the right eye. She was treated with warfarin anticoagulant therapy for recurrent deep vein thrombosis and pulmonary embolism. Haematological examination carried out because of the presence in family members of deep vein thrombosis and mesenteric vascular thrombosis and confirmed heterozygous FII prothrombin mutation and C677T methylenetetrahydrofolate reductase (MTHFR) gene mutation in homozygous form in the patient's son showed a heterozygous form of C677T MTHFR with a physiological level of homocysteinemia. During the eye examination, the CVA of the right eye was found to be 0.63 with a correction of -0,75 Dcyl ax 180°. We diagnosed subhyaloid haemorrhage biomicroscopically and by SD-OCT, located just above the fovea (Figure 1 A).
Figure 1. Subhyaloid haemorrhage in the right eye of patient No. 1 receiving warfarin therapy (A), subhyaloid haemorrhage spontaneously gradually resolved (B-I) 
Patient No. 2, a 50 - year-old woman suffering from type 2 diabetes mellitus treated with oral antidiabetics, arrived at our workplace for 14 days due to immobile cobwebs in the visual field of the right eye. The initial CVA of the right eye was 0.63 with a correction of −0.50 D = −1.25 Dcyl ax 80°. Biomicroscopic examination revealed subhyaloid haemorrhage (Figure 2 A), which was subsequently confirmed by SD-OCT (Figure 2 G).
Figure 2. Subhyaloid haemorrhage in the right eye of patient No. 2 (A), immediately after Nd:YAG laser hyaloidotomy (B, C), one week after Nd:YAG laser hyaloidotomy (D), fluorescein angiogram showing retinal neovascularization (arrowheads) (E), stabilization diabetic retinopathy after 27 months (F). Linear transfoveolar horizontal optical coherence tomography scan showed subhyaloid haemorrhage (G), linear horizontal inferoextrafoveolar optical coherence tomography scan demonstrating a residual sub-hyaloid cavity (arrowhead) after 1 month (H) and linear horizontal transfoveolar optical coherence tomography scan after 27 months of Nd:YAG laser hyaloidotomy (I) 
Patient No. 3, a 51 -year-old healthy man, was examined for impaired left eye vision lasting 1 month, which was preceded by greater physical exertion. CVA was 0.16 with correction +1.0 D = −0.5 Dcyl ax 170°. Upon fundoscopic examination, erythrocytes were scattered in the vitreous of the left eye, and the posterior pole area was covered with a domed haemorrhage (Figure 3 A). SD-OCT examination confirmed the localization of bleeding under the ILM in the macula (Figure 3 B, C).
RESULTS
In patient No. 1 a conservative approach was chosen, anticoagulant therapy was adjusted, and a lying position on the right side was recommended. Nd: YAG laser hyaloidotomy of the posterior vitreous membrane was not indicated, due to the upper juxtafoveolar localization of bleeding. The patient underwent regular weekly eye examinations for possible indication of Nd: YAG laser hyaloidotomy of the posterior vitreous membrane and/or pars plana vitrectomy (PPV) if preretinal haemorrhage shifted to the centre of the macula. Complete resorption of bleeding occurred within 4 months (Figure 1 B-I), the CVA of the right eye was adjusted to 1.0. The patient's follow-up monitoring lasted a total of 32 months.
Patient No. 2 underwent Nd: YAG laser hyaloidotomy of the posterior vitreous membrane. The selected energy of the Nd: YAG laser was in the range of 2.4–3.8 mJ. Immediately after the procedure, subhyaloid hemorrhage was evacuated to the vitreous space (Figure 2 B, C). One week after the procedure (Figure 2 D), the CVA improved to 0.8. Retinal neovascularizations were identified as the source of preretinal haemorrhage and were confirmed by fluorescence angiography (Figure 2 E). Subsequently, focal laser photocoagulation was performed and at the same time clinically significant macular oedema was treated with a laser. In the next follow-up period, for 27 months, the irregularity on the ocular background of the right eye was stabilized (Figure 2 F). After complete resorption of preretinal haemorrhage, a residual subhyaloid pocket was captured on SD-OCT (Figure 2 H), with no evidence of epimacular membrane development in the next follow-up period (Figure 2 I).
Patient No. 3 was indicated for surgical treatment by the method of suture-free 25 -Gauge PPV with ILM peeling and short-term gas tamponade with 20 % hexafluorosulfide. The patient underwent the planned operation within a week of making the eye diagnosis. The removed ILM was sent for ultrastructural morphometric examination. Morphometric analysis showed a variable ILM thickness of 2.70 ± 1.58 μm. On the retinal side of the ILM, there were signs of organizing and resorbing hematoma with abundant involvement of pigmented macrophages and elements of fibroblastic nature (Figure 3 E-H). In the postoperative period, the CVA of the left eye improved to 1.0 and the irregularity on the retina also improved (Figure 3 D). The total follow-up period with the patient was three months.
Figure 3. Colour fundi photographs of sub-internal limiting membrane (ILM) haemorrhage in the left eye of patient No. 3 (A), linear transfoveolar vertical optical coherence tomography scan showed sub-ILM haemorrhage (B), linear horizontal juxtafoveolar optical coherence tomography scan revealed dome-shaped haemorrhages between elevated thickened ILM and retinal nerve fibre layer (C), after 25-G pars plana vitrectomy combined with internal limiting membrane peeling (D). Morphometric and ultrastructural analysis of the excised tissue, high-power view of section reveals ILM with adjacent cells (F: fibroblast, L: lymphocyte, M: hemosiderin-laden macrophages), variable thickness ILM (2,70 µm ±1,58 µm), on the retinal surface of the ILM signs of resorbing and organizing hematoma with the participation of abundant pigmented macrophages and elements of fibroblastic nature, magnification 5000x with a scale of 1 µm in the shot (E-G). Light microscopic analysis of semi-thin section showed ILM with adjacent pigmented macrophages, magnification 60x (H) 
No complications of the treatment used were reported in patients. The results are summarized in Table 1.
1. Results of various treatments for premacular bleeding and bleeding below the inner limiting membrane 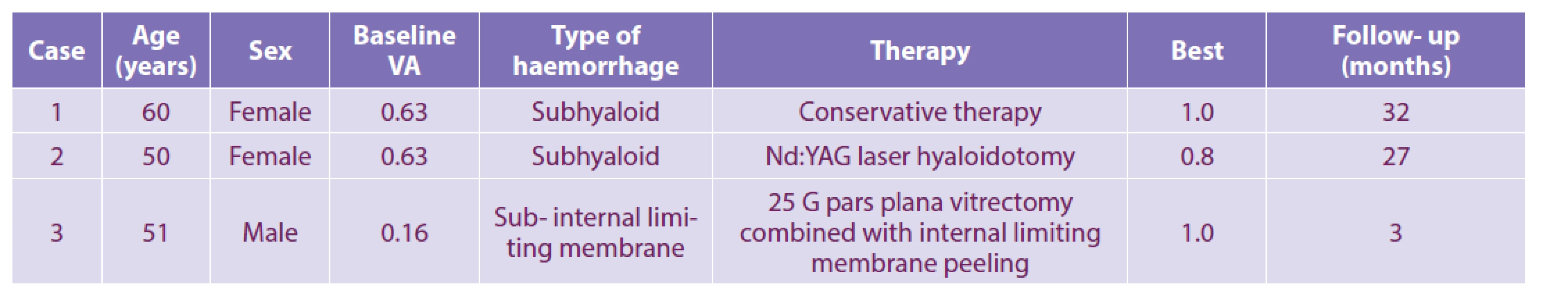
VA: visual acuity DISCUSSION
Subhyaloid haemorrhage or haemorrhage below the inner limiting membrane of the retina in the posterior pole of the eye can be a manifestation of a number of diseases. It is usually accompanied by a sudden loss of CVA.
To rapidly restore visual function, we can use the Nd: YAG method of laser hyaloidotomy of the posterior vitreous membrane accompanied by the release of subhyaloid haemorrhage into the vitreous space. The set laser energy should not exceed 10.5 mJ, due to a higher risk of complications in terms of the development of a macular hole or retinal rupture with the risk of subsequent retinal detachment [1]. We performed Nd: YAG laser hyaloidotomy of the posterior vitreous membrane in patient No. 2 of our test group. The energy of the Nd: YAG laser ranged from 2.4 to 3.8 mJ. The timing of Nd: YAG laser hyaloidotomy of the posterior vitreous membrane should be in the range of 1–3 weeks from the onset of the problem [1], and was preform on our patient in 2 weeks. Before performing a Nd: YAG laser hyaloidotomy of the posterior vitreous membrane, it is necessary to preform SD-OCT examination to accurately determine the location of bleeding (subhyaloid or bleeding under ILM). Laser disruption of the posterior vitreous membrane is performed at the lower edge of subhyaloid haemorrhage, in the places where the posterior vitreous membrane is most arched and there is a larger interval of distance from the retinal surface [2].
After complete evacuation of preretinal haemorrhage after Nd: YAG laser hyaloidotomy of the posterior vitreous membrane, a persistent subhyaloid cavity was detected during SD-OCT examination in patient No. 2. Sabella et al. (2010) documented its healing after signs of Nd: YAG laser membranotomy for bleeding under the ILM without signs of damage to the retinal nerve fibre layer [3]. Zou et al. [4] reported a non-healing preformed hole in the ILM and epimacular membrane formation after performing a Nd: YAG laser membranotomy for bleeding under the ILM. In our patient, we did not detect the formation of an epiretinal membrane during biomicroscopy or SD-OCT.
In patient No. 1, this procedure was not indicated because the lower edge of the haemorrhage reached the upper edge of the fovea. Due to the risk of possible retinal damage by invasive procedure and good CVA, the conservative procedure was chosen. Particularly in subhyaloid haemorrhage, depending on the positioning of the head, it is possible through the effect of gravity to achieve the displacement of blood in the retrohyaloid space. In patient No. 1, this procedure resulted in the transfer of the haemorrhage out of the axis of vision. However, in bleeding below the ILM, spontaneous bleeding resorption is usually prolonged and the possibility of toxic damage to retinal nerve cells by hemoglobin breakdown products cannot be ruled out. Gibran et al. and Krásnik et al. [5,6] reported that spontaneous absorption of haemorrhage under the ILM is associated with a retinal toxicity, premature traction membrane formation, and proliferative vitreoretinopathy.
Surgical peeling of the ILM and aspiration of macular bleeding during suture-free 25 -Gauge PPV can eliminate the risk of toxic retinal nerve cell damage. During the operation in our patient No. 3, the ILM was peeled off and sent for histological and morphometric examination. In 2007, Gibran et al. and in 2013 Hua et al. presented histological results of peeling ILM during PPV for bleeding under ILM. [5.7]. On the retinal side of the peeled ILM, there were present macrophages with phagocytosed hemosiderin and cells similar to retinal pigment epithelial cells, which are responsible for the formation of the glial membrane. No cells were found on the vitreous side of the peeled ILM. These histological findings were also confirmed in patient No. 3. Tissue injury flushes inflammatory monocytes, tissue macrophages, fibroblasts, inflammatory mediators, and growth factors to repair damaged tissue [8]. The histological finding of peeled ILM in patient No. 3 confirms the ongoing repair processes of the injured tissue.
We believe that the cause of bleeding under the ILM in this patient was the Valsalva manoeuvre. During exercise, there is an increase in abdominal and intrathoracic pressure, which results in an increase in venous pressure and may cause retinal capillary bleeding under the ILM. If the bleeding is massive, it can cause cracks in the ILM and blood can further penetrate into the subhyaloid or even vitreous space [9,10]. In our cohort, we did not observe any complications associated with the surgical treatment of bleeding under the ILM by suture-free 25-G PPV or Nd: YAG laser hyaloidotomy of the posterior vitreous membrane.
CONCLUSION
The choice of treatment for preretinal bleeding (PPV, Nd: YAG laser hyaloidotomy or conservative procedure) depends on input parameters such as CVA, type and extent of bleeding and, last but not least, the overall health of the patient. Nd: YAG laser hyaloidotomy is a fast and effective method leading to the restoration of the patient's visual function. Surgical aspiration of bleeding under ILM with peeling of ILM helps to remove iron and hemosiderin deposits, thus minimizing the risk of secondary retinal membranes and toxic damage to retinal nerve cells.
The authors declare that the origin and topic of the professional communication and its publication is not in conflict of interest and is not supported by any pharmaceutical company. The work was not assigned to another journal or published elsewhere.
The study was supported by a grant from Charles University Progress Q25.
Received: 25 August 2021
Accepted: 30 October 2021
Available on-line: 25 November 2021MUDr. Magdalena Kováčová
Oční klinika, 1. lékařská fakulta, Univerzita Karlova a Všeobecná fakultní nemocnice v Praze
U nemocnice 2
128 08 Praha 2
E-mail: magdalena.kovacova@vfn.cz
Sources
1. Kirwan RP, Cahill MT. Nd:YAG laser hyaloidotomy for Valsalva pre-macular haemorrhage. Ir J Med Sci. 2011 Sep;180(3):749-752.
2. Karagiannis D, Kontadakis GA, Flanagan D. Nd:YAG laser for preretinal hemorrhage in diabetic retinopathy. Am J Ophthalmol Case Rep. 2018 Jan 12;10 : 8-9.
3. Sabella P, Bottoni F, Staurenghi G. Spectral-domain OCT evaluation of Nd:YAG laser treatment for Valsalva retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010 Apr;248(4):599-601.
4. Zou M, Gao S, Zhang J, Zhang M. Persistent unsealed internal limiting membrane after Nd:YAG laser treatment for Valsalva retinopathy. BMC Ophthalmol. 2013 Apr 16;13 : 15.
5. Gibran SK, Kenawy N, Wong D, Hiscott P. Changes in the retinal inner limiting membrane associated with Valsalva retinopathy. Br J Ophthalmol. 2007 May;91(5):701-702.
6. Krásnik V, Stefanicková J, Strmen P, Kusenda P. Krvácania pod vnútornú hraničnú membránu sietnice liečené pars plana vitrektómiou [Sub - internal Limiting Membrane Haemorrhage Treated by Pars Plana Vitrectomy]. Cesk Slov Oftalmol. 2012 Oct;68(4):135 - 739.
7. Hua R, Liu LM, Hu YD, Zhou Y, Chen L. Combine intravitreal bevacizumab with Nd: YAG laser hyaloidotomy for Valsalva pre-macular haemorrhage and observe the internal limiting membrane changes: a spectralis study. Int J Ophthalmol. 2013 Apr 18;6(2):242-245.
8. Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016 Mar 15;44(3):450-462.
9. García Fernández M, Navarro JC, Castaño CG. Long-term evolution of Valsalva retinopathy: a case series. J Med Case Rep. 2012 Oct 10;6 : 346.
10. Couperus K, Angel S, Kim N, Flugga K, Perreault M. A unique case of Valsalva retinopathy: preretinal hemorrhage identified on bedside ultrasound. J Emerg Med. 2021 Feb;60(2):220-222.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology
2021 Issue 6-
All articles in this issue
- OČNÍ LÉKAŘ KAREL KUBĚNA
- Životné jubileum MUDr. Teodora Streichera
- PHOTOREFRACTIVE SURGERY WITH EXCIMER LASER AND ITS IMPACT ON THE DIAGNOSIS AND FOLLOW-UP OF GLAUCOMA. A REVIEW
- OCT ANGIOGRAPHY, VISUAL FIELD AND RNFL WITH VARIOUS MEDICATIONS IN HYPERTENSIVE GLAUCOMAS
- TREATMENT OPTIONS FOR PREMACULAR AND SUB-INTERNAL LIMITING MEMBRANE HEMORRHAGE
- COMPARISON OF OPTICAL BIOMETERS ARGOS AND IOL MASTER 700
- PARANEOPLASTIC OPTIC NEUROPATHY AS AN INITIAL CLINICAL MANIFESTATION OF SMALL CELL LUNG CANCER. A CASE REPORT
- INTRAOCULAR LYMPHOMA WITH RETROBULBAR INFILTRATION. A CASE REPORT
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- OCT ANGIOGRAPHY, VISUAL FIELD AND RNFL WITH VARIOUS MEDICATIONS IN HYPERTENSIVE GLAUCOMAS
- COMPARISON OF OPTICAL BIOMETERS ARGOS AND IOL MASTER 700
- TREATMENT OPTIONS FOR PREMACULAR AND SUB-INTERNAL LIMITING MEMBRANE HEMORRHAGE
- INTRAOCULAR LYMPHOMA WITH RETROBULBAR INFILTRATION. A CASE REPORT
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


