-
Medical journals
- Career
PRESBYOPIA MANAGEMENT WITH DIFFRACTIVE PHAKIC POSTERIOR CHAMBER IOL
Authors: G. R. Bianchi
Authors‘ workplace: Buenos Aires, Argentina. ; Centro Panamericana ; Blas Parera 4201. B1636CSS - OLIVOS ; Clínica de Ojos Dr. Nano.
Published in: Čes. a slov. Oftal., 76, 2020, No. 5, p. 211-219
Category: Original Article
doi: https://doi.org/10.31348/2020/30Overview
Objective: To evaluate safety and refractive efficiency after posterior chamber diffractive implantable phakic contact lens (IPCL) surgery.
Material and Methods: A prospective non-randomized case-series study was performed on 54 myopic eyes of 27 patients who had undergone diffractive IPCL surgery. Corneal endothelial cell density (ECD), central corneal thickness (CCT), intra-ocular pressure (IOP), vault, uncorrected distance (UDVA), spherical equivalent (SE) and defocus curve, were all evaluated twelve months after surgery. The presence of cataracts was evaluated by slit-lamp during a postoperative follow-up.
Results: Mean age was 47 ± 2.62 years-old. Mean SE decreased, from -5.95 ± 2.56 D in a pre-operative stage, to -0.25 ± 0.25 D twelve months after surgery. Achieved UDVA was 20/20 in 24.1% of all cases, 20/25 in 74.1% of them, and 20/32 in all remaining cases. No eyes suffered lost lines of vision. The binocular defocus curve was 0.06 ± 0.05 logMAR for a -3.0 D of defocus; 0.11 ± 0.04 logMAR for a -1.5 D of defocus, and 0.08 ± 0.03 logMAR for a 0 D of defocus. Twelve months after surgery, mean ECD had decreased by 1.43 %, whereas mean CCT had increased by 0.06 %, without any significant statistical difference (p = 0.28 and p = 0.93 respectively). No difference (p: 0.86) in the vault was observed at 6 months vs.12 months, as well as between IOP measurements (p = 0.22). There were no non-intra or postoperative complications, and, specifically, no cataracts developed either.
Conclusions: Diffractive IPCL was implanted safely. Corneal endothelial CD, CCT, vault, and IOP remained stable twelve months after surgery. Visual acuity for distance, intermediate and near sight were achieved without spectacles.
Keywords:
refractive surgery – Presbyopia – phakic intra-ocular lens – posterior chamber
INTRODUCTION
Presbyopia is an increasing problem in adults aged over forty years. As a result, correction of presbyopia has become a developing and fast growing part of refractive surgery. Corneal procedures have their limitations in connection with the structure of the cornea and the overall size of ammetropia [1-3]. It is possible to use multifocal intraocular lenses (IOL) [4]. However, extraction of a clear lens in myopic eyes is controversial due to the increased risk of retinal complications [5-7].
A phakic intraocular lens (pIOL) can correct high myopia and hyperopia with the advantage of reversibility, stability of correction and preservation of accommodation [8-14]. It is also expected that higher quality of vision in high ammetropias can be attained with the aid of pIOL in comparison with keratorefractive surgery [15-18]. Implantation of a pIOL into the anterior chamber is linked with complications, which have been described in a series of studies [19-21]. A phakic intraocular contact lens which can be implanted into the posterior chamber, termed an IPCL (Care Group, India), has demonstrated its safety and efficacy in correcting myopia and myopic astigmatism [22-24]. A new model of the lens, the “diffractive IPCL V 2.0”, represents an option for correcting presbyopia. The aim of this article is to assess the safety and refractive effectiveness of the diffractive IPCL V 2.0 in patients with myopia or myopic astigmatism and presbyopia.
METHODS
Conception of study
The safety and efficacy of implantation of a diffractive IPCL was assessed in a prospective non-randomised trial. The operations were performed from February 2018 to August 2018, with subsequent two-month observation. The study was in accordance with the principles of the “Helsinki declaration” and with the consent of the ethical commission of the “Clínica de Ojos Dr. Nano”. Written consent was obtained from all patients after provision of complete information about the characteristics and risks of the surgical procedure.
Criteria for inclusion and exclusion of patients
Inclusion criteria. Patients aged between 40 and 55 years with myopia, with or without astigmatism, and with stable refraction for a minimum period of one year were included in the study.
Exclusion criteria. All patients with cataract, patients in whom endothelial cell density (ECD) of the cornea was less than 2 000 cells/mm2, or who had an anterior chamber depth (ACD) of less than 2.8 mm were excluded from the study; also excluded were patients with a history of glaucoma, with glaucoma, or who had undergone retinal surgery. In addition, patients with a corneal pathology (dystrophy, degeneration or trauma, which could influence corneal transparency) were excluded. In the current series, patients with hypermetropia were also excluded.
Preoperative examination and assessment parameters
All the patients underwent a complete ophthalmological baseline examination. The patients’ age and sex was recorded. Preoperative assessment of the cornea (for the purpose of detecting regular or irregular astigmatism) and measurement of ACD was performed with the aid of a Pentacam instrument (Oculus, Wetzlar, Germany). Pentacam was also used to measure pupil diameter under scotopic conditions. This information was used for the selection of the optic zone of the IPCL. The size of the IPCL was determined on the basis of horizontal white-to-white distance (IOL-Master 500®; Carl Zeiss, Germany).
In all cases the goal was emmetropia. Before the surgical procedure and also twelve months afterwards, manifest spherical equivalent (SE) and astigmatism were assessed. Postoperative uncorrected distance visual acuity (UDVA) was compared with preoperative corrected distance visual acuity (CDVA) (Snellen charts). Twelve months after the surgical procedure, the logarithm of the minimal angle of resolution (logMAR) was calculated in order to obtain the defocus curve (addition from -4.0 to +2.0 D).
The size of optimal addition was calculated with the aid of our own nomogram on the basis of patient age and the degree of myopia (Table 1). A preoperative subjective evaluation of visual acuity with correction was conducted on all eyes. Two variants were available.
1. Nomogram for selection of best presbyopic addition (ADD) for each eye according to age, degree of myopia, condition of dominant and non-dominant eye and patient tolerance (variant A and variant B). Dioptre (D) 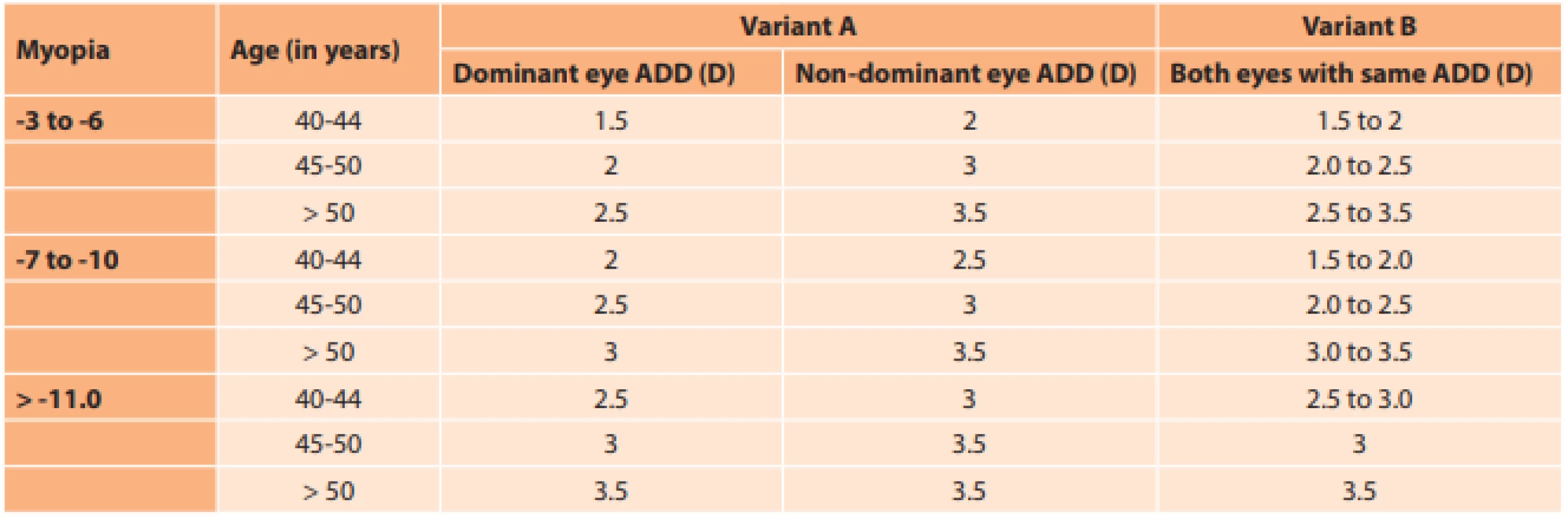
Selection of addition
Variant A: A lens with the highest tolerated addition was implanted in the dominant eye. This addition was increased by 0.50 to 1.0 D, as stated in table 1, and used in the non-dominant eye as “mixed addition”, which in the following years, upon increasing presbyopia, enables the patient to avoid the necessity of wearing glasses for near vision.
Variant B: If the difference between the dominant and non-dominant eye was not tolerated before surgery, identical addition was used for both eyes.
The highest level of addition, which could be subjectively tolerated, was selected according to age and degree of myopia.
ECD of the cornea and CCT were determined preoperatively and twelve months after the surgical procedure by specular endothelial microscope (TOMEY EM4000). Intraocular pressure (IOP) was measured before the procedure, 1 and 7 days afterwards, and subsequently one and twelve months after the procedure by Goldmann tonometer. Iridotomy was not performed before implantation of the IPCL. Arching of the IPCL was assessed at 6 and 12 months after surgery by an ultra-bio microscope AvisoTM; Quantel Medical.
If astigmatism was > 1.0 D, a toric diffractive IPCL V2.0 was used. Complications were evaluated during the course of the operation and afterwards, especially for the purpose of detecting signs of onset of cataract (with the aid of a slit lamp, always by the same observer), in accordance with the classification system Lens Opacities Classification System (LOCS) classification III.
Properties of diffractive IPCL V2.0 (official brochure on website http://caregroupiol.com/products/phakic-lenses/ipcl/)
The IPCL is a single-piece, foldable, hydrophilic acrylate injectable pIOL for the posterior chamber, which is available in Europe. It is inserted behind the iris, in which its haptic part should rest in the ciliary body. It is implanted by means of a 2.8 mm corneal incision. The construction of the lens incorporates 6 haptics for increasing stability. It has two openings in the peripheral part of the upper zone for reducing dazzling and halo effects, and four openings outside the optic zone. Version V2.0 has a central conical opening (380 µm) in order to facilitate circulation of the chamber fluid. The thickness of the IPCL is 80 µm. It is designed in such a manner as to correct myopia within the range of -1.00 to -33.00 dioptres (D) and hyperopia from +1.0 to +15.0 D. It has an aspherical optic zone with zero aberration. The diameter of the optic part is within the range of 5.75 to 6.20 mm, and the total size is from 11.0 mm to 14.00 mm (in gradations of 0.25 mm). The diameter of the optic part is available in variants of 6.50, 6.80, 7.20 or 7.50 mm, according to the patient’s pupil size. This lens has diffractive refractive technology (trifocal optic design), with concentric rings to support addition for near vision (ADD) from +1.5 to +4.0 (with gradations of 0.5 D) and intermediary addition of +2.1 D. The diffractive zone has an angle inclination, thanks to which light dispersion is reduced to 8 % or less. The progression of the angle inclination is within the range from 6° in the centre to 65° on the periphery of the lens. The angles of this inclination decrease from the centre to the periphery; beginning at 1.8 µm in the centre and reaching 90 µm on the periphery.
In the current study, the selection of the optic zone and the diameter for each eye was performed with the use of an online IPCL calculator (http://www.ipcliol.com/). All the used lenses were of a standard size (adjustment of size was not requested).
Description of surgical technique: steps and tips.
All the operations were performed by the same surgeon. Viscoelastic materials were not used whatsoever, as published previously [24]. An incision (at an angle of 45 degrees) was made under local anaesthesia of the cornea, using a 20G V-knife. The anterior chamber was sustained by an infusion with the aid of a bimanual 23G irrigation/aspiration (I/A) cannula. After the first incision, a second 2.8 mm incision was made (at an angle of 130 degrees). There followed implantation of the phakic lens, the anterior chamber was sustained by balanced saline solution (BSS). The lens was carefully unfolded by I/A cannula and the haptics were placed behind the pupil at a position of 3 and 9 o’clock. Perioperative local treatment was identical in all cases; beginning three days before the operation with the application of 0.5 % gatifloxacin and 0.09 % bromfenac four times per day, after surgery the patients continued in this treatment, with the addition of one drop of 0.05 % difluprednate four times per day for a period of four weeks.
STATISTICAL PROCESSING
The descriptive statistical results are presented as the median value, standard deviation (SD) and range. Normality of the data was checked with the aid of a Kolmogorov-Smirnov test. A Wilcoxon test was conducted to compare the differences between central corneal endothelial cell density, CCT (baseline value vs. 12 months after surgery) and arching (6 vs. 12 months after surgery). An ANOVA analysis (single-factor) was used for evaluation of IOP. P values of lower than 0.05 were considered statistically significant. The reliability coefficient (R2) for assessment of the correlation between the intended and actually attained change of spherical equivalent (SE) was calculated as a component of the linear regression analysis. A statistical analysis was conducted with the aid of the software XLMiner Analysis ToolPak (Frontline Systems Inc.). The data is available from the organisation “Clínica de Ojos Dr. Nano”.
RESULTS
A total of 27 patients were operated on (54 eyes, of which a toric IPCL V.20 was used on 13 eyes). The mean age of the patients was 47 ± 2.62 years (43-53). The ratio of women to men was 15/12. All the surgical procedures were performed without any complications during the course of the operation. Cataract did not originate in any of the eyes within twelve months after surgery.
Safety of cornea, IOP and arching
Twelve months after the surgical procedure, average ECD was reduced by 1.43 % (38.74 cells/mm2) and mean CCT increased by 0.06 % (0.35 µm), without significant statistical differences (all the values are presented in table 2). The IOP values remained similar at all follow-up examinations, without a statistically significant difference (Table 2). One day after the surgical procedure a slight increase was observed, but the IOP values always fluctuated within a normal range. In the period of 6 to 12 months after surgery, average arching decreased by 0.55 % (2.89 µm), without statistically significant differences (p = 0.86), as can be seen in table 2.
2. Median values, standard deviation [range] from endothelial cell density (ECD), central corneal thickness (CCT), arching and intraocular pressure (IOP) were compared in different time points. A statistically significant difference was observed (p < 0.05). ![Median values, standard deviation [range] from endothelial cell density (ECD), central corneal thickness (CCT), arching and
intraocular pressure (IOP) were compared in different time points. A statistically significant difference was observed (p < 0.05).](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/6fc9e410e9dbe3f4eb7fe20a39a96a2b.png)
None of the eyes lost a single row of visual acuity, in 59.6 % of eyes visual acuity was unchanged, 40.4 % of eyes showed an improvement (gain of 1 row of Snellen chart), as can be seen in graphs 1 and 2. Mean postoperative SE was -5.96 ± 2.10 D (-3.12 to -12.50), which twelve months after the surgical procedure was reduced to -0.25 ± 0.25 D (+0.25 to -0.75). Graph 3 presents the correlation coefficient (R2 : 0.93), which states the strength of the correlation between the intended and actually attained change of SE. The spherical effectiveness of refractive precision is illustrated in Graph 4. Postoperative manifest astigmatism was -0.77 ± 0.59 /0 to -2.5). The attained value of astigmatism and the preoperative comparison are illustrated in Graph 5. Good results were attained for various additions of defocusing, as can be seen in Graph 6. Mixed addition (variant A) was used for 21 patients. In the remaining patients addition was the same for both eyes (variant B).
1. Uncorrected distance visual acuity (UDVA). Cumulative percentage of eyes which attained the specified levels of postoperative uncorrected distance visual acuity (UDVA) in comparison with the cumulative percentage of eyes that attained the specified levels of preoperative corrected distance visual acuity (CDVA) following the implantation of a diffractive ICPL 
2. Changes of corrected distance visual acuity (CDVA) 12 months after surgery. Refractive precision of spherical equivalent. Spherical equivalent attained in 6 months is connected with percentage of eyes 
3. Intended vs. attained spherical equivalent. Correlation between intended and attained change of spherical equivalent is documented by reliability coefficient (R2) 
4. Refractive precision of spherical equivalent 12 months after surgery 
5. Acquired refractive astigmatism 12 months after surgery and preoperative comparison 
6. Defocus curve (binocular) 6 months after surgery (n = 27 patients; logMAR) 
DISCUSSION
The author of this study has experiences of the implantation of IPCL lenses for correction of patients with high myopia dating back to 2015. This study refers to the first results of the use of IPCL V2.0. Its safety and efficacy was assessed 12 months after the surgical procedure according to various aspects. These aspects are discussed below.
Presbyopia is an unresolved problem, and various surgical and non-surgical variants exist for its correction. Refractive procedures with a phakic or pseudophakic IOL are focused on improving the quality of vision and lives of patients in their fourth decade of life. Laser refractive procedures are not recommended for patients with presbyopia with a thin cornea and high myopia. The number of surgical pr ocedures with a posterior chamber phakic IOL is increasing, and some studies emphasise their advantages in comparison with refractive interventions on the cornea with regard to excluding complications such as postoperative ectasia [2,25,26], refractive regression, high aberration index [3,17,18,27], problems with healing of surgical wound on cornea [28,29] and pathology of the ocular surface (dry eye, neuropathic pain) [30,31]. Treatment of presbyopia in the myopic population is not easy. It is natural that the majority of people with myopia have good near vision also without the aid of glasses. After the age of forty they must change their habits (take off glasses, cease using contact lenses, start using multifocal glasses). Even individual with myopia who for a certain time after the surgical procedure have good results in terms of distance and near vision usually eventually start to need glasses again due to presbyopia.
Implantation of a pIOL is a reversible refractive procedure, which preserves accommodation with minimal induction of higher order aberrations in comparison with photo-ablative procedures on the cornea [18]. The posterior chamber pIOL has undergone a significant improvement. However, to date there are no extensive studies on the model of the IPCL. In two studies [22,23], the authors assessed the previous model, the IPCL V1.0 (which required the performance of iridotomy). With a few small differences, both studies presented the best refractive results without complications in myopic correction and myopic astigmatism. A study has also been published on the IPCL V2.0 (new model with central opening), which presented safe and effective results [24]. The current study is the first to present the results of the diffractive IPCL V2.0 with emphasis on questions of safety and the visual result.
Question of safety: Intraocular pressure, cornea (endothelial cell density and central corneal thickness) and arching.
As is evident from the results of the current study, postoperative IOP remained the same over time, similarly as in the previously presented study relating to IPCL with the same platform (with central opening), which was performed by the same surgical technique, entirely without the use of viscoelastic materials [24]. This surgical method may have an “extra value” in the sense that it excludes potentially high values of IOP after surgery, which are connected with insufficient removal of viscoelastic material [21,32]. In addition, the safety of the procedure of implantation of the IPCL without viscoelastic materials for the cornea was assessed by means of measurement of corneal ECD and CCT. Both parameters were stable and without significant statistical differences twelve months after the surgical procedure. A similar result was attained in the previous study, although only within the framework of subsequent six-month observation of the model IPCL V2.0 [24]. With the previous platform of IPCL (V1.0), which was evaluated by different authors, analogous safety results were attained upon the use of a surgical technique with the use of viscoelastic materials [22,23]. For this reason, and for the purpose of confirming this aspect, it is essential to conduct a multicentric study comparing the existing technique with surgical procedures conducted with the aid of viscoelastic materials.
Central arching is the distance between the side of the IPCL and the anterior side of the patient’s own lens. This is an important safety parameter, which at the same time is connected with the refractive result. If the size (diameter) of the IPCL is not selected correctly, a number of problems may appear – in the case of lower arching there is a greater risk of the development of cataract. A safe value of arching must be between 250 and 750 µm; in the opposite case there is an increased risk of the occurrence of cataract. If arching reached values close to 1 000 µm, the lens had to be explanted [21,33,34]. Arching observed in this study was stable over time, without a statistically significant difference in the subsequent period of 6 to 12 months, which emphasises the postoperative stability and safety of the IPCL with regard to the fact that cataract did not develop in any of the cases.
Visual acuity
Refractive effectiveness was demonstrated by a reduction of SE following the surgical procedure and the reliability coefficient, which approached 1 (R2: 0.93). No decrease of visual acuity was recorded in any of the eyes; the UDVA value was similar to preoperative CDVA, and the majority of cases attained a value of 20/25 or better. Only 1.9 % of all eyes recorded an UDVA value of 20/32. The good refractive result was similar to the results that were published previously [22-24], which confirms the information relating to the effectiveness of this lens in adjusting ammetropias. The obtained defocus curve furthermore provides us with information on how well patients are able to see to various distances without the aid of glasses. The data confirms very good results for distance and near vision, with a slight decrease in medium distance vision twelve months after the surgical procedure. The normogram used in this study was designated for ensuring long-term independence of glasses (throughout the entire duration of the presbyopic period), thanks to the use of the highest tolerated additions for all patients. In the majority of cases the mixed variant was selected. The results of both strategies (mixed vs. identical addition for both eyes) were not compared; however, this concerns a further aspect which will be interesting to evaluate in future.
The diffractive model of the IPCL appears to be a very attractive choice for patients aged between 40 and 50 years. However, it is necessary to determine its effectiveness in a larger number of patients and over the course of a longer observation period. The main aspect for solution is near vision in operated myopic patients. It is well known that patients suffering from short-sightedness seek assistance for presbyopic symptoms later than others. Nevertheless, these individuals also remove their glasses when performing close-up work. Within the framework of the current series, the median age reached 47 ± 2.62 years (43-53 year old patients). The maximum subjective measured accommodation amplitude decreased by approximately 0.6 D per year of age [36], and some studies confirm that no relationship exists between refractive error and accommodation amplitude [36,37]. Furthermore, L.M. Abraham et al. come to the conclusion that a higher accommodation amplitude exists among patients suffering from short-sightedness in the age group of 35 to 44 years in comparison with patients with emmetropia and hyperopia. After patients have reached the age of 44 years, accommodation amplitude converges towards analogous values [37]. The total volume of residual accommodation from the existing publications and the total effect produced by the diffractive IPCL is therefore not clear. It is possible that the diffractive IPCL is in interaction with the patient’s own lens, by which it supports the patient’s residual accommodation [38]. Patients do not make use of the diffractive component of the lens while they still maintain accommodation, but as soon as presbyopia develops within them, their brain will automatically select this upon reading, and they will not be necessitated to use glasses. For an assessment of how long these patients may remain independent of glasses, a longer observation period will undoubtedly be necessary.
In the past authors evaluated posterior chamber phakic lenses for addressing presbyopic problems, but with the use of monofocal lenses and monovision. M. Takahashi et al. [39] worked with a cohort of 21 patients with a mean age of 45.0 ± 3.8 years (within the range of 40 to 53 years) upon implantation of a Vision Implantable Collamer Lens (ICL). Thanks to the planned under-correction in the non-dominant eye, they attained acceptable results 6 months after surgery. A similar study with monovision and implantation of an ICL was published by K. Kamiya et al. [40], and related to 17 patients with a mean age of 46.1 ± 4.2 years (within the range of 40 to 53 years). After a 6 month observation period they attained independence of glasses without complications. Following a rigorous study of their limitations and problems, it is possible to state that monovision is a good variant for treating presbyopia [41].
The possibilities of intraocular lenses correcting presbyopia are increasing, although their scientific and objective evaluation is not easy, as discussed by J.L. Alio in an interesting editorial contribution [42]. A new insight and strength of this report is that it is the first study to analyse the use of a diffractive ICPL as a new variant for correcting this common problem. A further original aspect is the information presented in Table 1, which contains a nomogram for the selection of a corresponding “mixed” addition, in which preoperative subjective refraction and potential future addition are taken into consideration. This aspect will nevertheless require a longer observation period in order to enable an assessment of how long these patients will be able to manage without glasses for correction of presbyopia. The study also included a description of the used surgical technique, although this was not its goal, with regard to the fact that the technique may influence the results. However, no comparison of techniques was conducted.
One of the limitations of this study is that aspects of quality of vision were not assessed, such as dazzling and contrast vision. An interesting study was published by Martínez-Plazou et al. [43], evaluating resulting dazzling in a procedure performed with the aid of another phakic contact lens for the anterior chamber (ICL, model V4c) with several similar characteristics as the IPCL, producing good results. A larger number of parameters of quality of vision were measured by Qin et al. [44] in a hypermetropic population, and here also good effectiveness was proven. However, the previous studies were conducted with the use of different lenses, which lacked the diffractive optics of the IPCL.
It shall therefore be necessary to assess these parameters in a future study on the diffractive IPCL V2.
CONCLUSIONS
This study confirms that twelve months after implantation of a diffractive IPCL with a surgical technique that does not use viscoelastic materials whatsoever, neither corneal ECD nor CCT were altered. After a period of 6 to 12 months after implantation of the IPCL, arching remained stable, within the framework of the safety parameters and without the onset of cataract. The obtained postoperative refraction was sufficiently good in order to attain independence of glasses, and corresponded with preoperative expectations for the spherical and toric model in the myopic population with presbyopia. To confirm the existing good results it will be necessary in future for studies to be conducted with a diffractive IPCL, with a longer observation period and by further surgeons.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
G. R. Bianchi
Clínica de Ojos Dr. Nano.
Centro Panamericana
Blas Parera 4201. B1636CSS – OLIVOS
Buenos Aires, Argentina
Sources
1. Perez-Santonja JJ, Bellot J, Claramonte P, Ismail MM, Alio JL. Laser in situ keratomileusis to correct high myopia. J Cataract Refract Surg. 1997;23 : 372–385.
2. Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Cataract Refract Surg. 2001;27 : 1796–1802.
3. Applegate RA, Howland HC. Refractive surgery, optical aberrations, and visual performance. J Refract Surg. 1997;13 : 295–299.
4. Rocha KM. Extended Depth of Focus IOLs: The Next Chapter in Refractive Technology? J Refract Surg. 2017;33 : 146–149.
5. Goldberg MF. Clear lens extraction for axial myopia. Posudek. Ophthalmology. 1987;94 : 571–582.
6. Barraquer C, Cavelier C, Mejia LF. Incidence of retinal detachment following clear-lens extraction in myopic patients. Retrospektivní analýza. Arch Ophthalmol. 1994;112 : 336–339.
7. Garrana RM, Azar DT. Phakic intraocular lenses for correction of high myopia. Int Ophthalmol Clin. 1999;39 : 45–57.
8. Sanders DR, Vukich JA, Doney K, Gaston M. US Food and Drug Administration clinical trial of the Implantable Contact Lens for moderate to high myopia. Ophthalmology. 2003;110 : 255–266.
9. Baikoff G, Arne JL, Bokobza Y, Colin J, George JL, Lagoutte F, Lesure P, Montard M, Saragoussi JJ, Secheyron P. Angle-fixated anterior chamber phakic intraocular lens for myopia of -7 to -19 diopters. J Refract Surg. 1998;14 : 282–293.
10. Alio JL, de la Hoz F, Perez-Santonja JJ, Ruiz-Moreno JM, Quesada JA. Phakic anterior chamber lenses for the correction of myopia: A 7-year cumulative analysis of complications in 263 cases. Ophthalmology. 1999;106 : 458–466.
11. Davidorf JM, Zaldivar R, Oscherow S. Posterior chamber phakic intraocular lens for hyperopia of +4 to +11 diopters. J Refract Surg. 1998;14 : 306–311.
12. Zaldivar R, Davidorf JM, Oscherow S. Posterior chamber phakic intraocular lens for myopia of -8 to -19 diopters. J Refract Surg. 1998;14 : 294–305.
13. BenEzra D, Cohen E, Karshai I. Phakic posterior chamber intraocular lens for the correction of anisometropia and treatment of amblyopia. Am J Ophthalmol 2000;130 : 292–296.
14. Menezo JL, Peris-Martinez C, Cisneros AL, Martinez-Costa R. Phakic intraocular lenses to correct high myopia: Adatomed, Staar, and Artisan. J Cataract Refract Surg. 2004;30 : 33–44.
15. Alio JL. Advances in phakic intraocular lenses: Indications, efficacy, safety, and new designs. Curr Opin Ophthalmol. 2004;15 : 350–357.
16. Malecaze FJ, Hulin H, Bierer P, et al. A randomized paired eye comparison of two techniques for treating moderately high myopia: LASIK and artisan phakic lens. Ophthalmology. 2002;109 : 1622–1630.
17. Nio YK, Jansonius NM, Wijdh RH, et al. Effect of methods of myopia correction on visual acuity, contrast sensitivity, and depth of focus. J Cataract Refract Surg. 2003;29 : 2082–2095.
18. Sarver EJ, Sanders DR, Vukich JA. Image quality in myopic eyes corrected with laser in situ keratomileusis and phakic intraocular lens. J Refract Surg. 2003;19 : 397–404.
19. Mastropasqua L, Toto L, Nubile M, Falconio G, Ciancaglini M. Long-term complications of bilateral posterior chamber phakic intraocular lens implantation. J Cataract Refract Surg. 2004;30 : 901–904.
20. Menezo JL, Peris-Martínez C, Cisneros-Lanuza AL, Martínez-Costa R. Rate of cataract formation in 343 highly myopic eyes after implantation of three types of phakic intraocular lenses. J Refract Surg. 2004;20 : 317–324.
21. Fernandes P, Gonzalez-Meijome J, Madrid-Costa D, Ferrer-Blasco T, Jorge J, Montés-Micó R. Implantable collamer posterior chamber intraocular lenses: a review of potential complications. J Refract Surg. 2011;27 : 765–776.
22. Vasavada V, Srivastava S, Vasavada SA, Sudhalkar A, Vasavada AR, Vasavada VA. Safety and Efficacy of a New Phakic Posterior Chamber IOL for Correction of Myopia: 3 Years of Follow-up. J Refract Surg. 2018;34 : 817–823.
23. Sachdev G, Ramamurthy D. Long-term safety of posterior chamber implantable phakic contact lens for the correction of myopia. Clin Ophthalmol. 2019;13 : 137–142.
24. Bianchi GR. Initial Results From a New Model of Posterior Chamber Implantable Phakic Contact Lens: IPCL V2.0. Med Hypothesis Discov Innov Ophthalmol. 2019;8 : 57–63.
25. Roberts C. The cornea is not a piece of plastic. J Refract Surg. 2000;16 : 407–413.
26. Torres RM, Merayo-Lloves J, Jaramillo MA, Galvis V. [Corneal biomechanics]. Arch Soc Esp Oftalmol. 2005;80 : 215–223.
27. Marcos S. Aberrations and visual performance following standard laser vision correction. J Refract Surg. 2001;17:S596–601.
28. Fatseas G, Stapleton F, Versace P. Role of percent peripheral tissue ablated on refractive outcomes following hyperopic LASIK. PLoS One. 2017;12:e0170559.
29. Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107 : 1235–1245.
30. Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. 2010;8 : 135–145.
31. Garcia-Zalisnak D, Nash D, Yeu E. Ocular surface diseases and corneal refractive surgery. Curr Opin Ophthalmol. 2014;25 : 264–269.
32. Senthil S, Choudhari NS, Vaddavalli PK, Murthy S, Reddy JC, Garudadri CS. Etiology and Management of Raised Intraocular Pressure following Posterior Chamber Phakic Intraocular Lens Implantation in Myopic Eyes. PLoS One. 2016;11:e0165469.
33. Pérez-Cambrodí RJ, Piñero DP, Ferrer-Blasco T, Cerviño A, Brautaset R. The posterior chamber phakic refractive lens (PRL): a review. Eye. 2013;27 : 14–21.
34. Sanchez-Galeana CA, Smith RJ, Sanders DR, et al. Lens opacities after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2003;110 : 781–785.
35. Richdale K, Bullimore MA, Sinnott LT, Zadnik K. The Effect of Age, Accommodation, and Refractive Error on the Adult Human Eye. Optom Vis Sci. 2016;93 : 3–11.
36. Schaeffel F, Wilhelm H, Zrenner E. Inter individual variability in the dynamics of natural accommodation in humans in relation to age and refractive errors. Journal of Physiol (Lond). 1993;461 : 301–320.
37. Abraham LM, Kuriakose T, Sivanandam V, Venkatesan N, Thomas R, Muliyil J. Amplitude of accommodation and its relation to refractive errors. Indian J Ophthalmol. 2005;53 : 105–108.
38. Petternel V, Koppl CM, Dejaco-Ruhswurm I, Findl O, Skorpik C, Drexler W. Effect of accommodation and pupil size on the movement of a posterior chamber lens in the phakic eye. Ophthalmology. 2004;111 : 325–331.
39. Takahashi M, Kamiya K, Shoji N, Kato S, Igarashi A, Shimizu K. Intentional Undercorrection by Implantation of Posterior Chamber Phakic Intraocular Lens With A Central Hole (Hole ICL) For Early Presbyopia. Biomed Res Int. 2018;10 : 6158520.
40. Kamiya K, Takahashi M, Takahashi N, Shoji N, Shimizu K. Monovision by Implantation of Posterior Chamber Phakic Intraocular Lens with a Central Hole (Hole ICL) for Early Presbyopia. Sci Rep. 2017;7 : 11302.
41. Khandelwal SS, Jun JJ, Mak S, Booth MS, Shekelle PG. Effectiveness of multifocal and monofocal intraocular lenses for cataract surgery and lens replacement: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257 : 863–875.
42. Alio JL. Presbyopic Lenses: Evidence, Masquerade News, and Fake News. Asia Pac J Ophthalmol (Phila). 2019;8 : 273–274.
43. Martínez-Plaza E, López-Miguel A, Fernández I, Blázquez-Arauzo F, Maldonado MJ. Effect of central hole location in phakic intraocular lenses on visual function under progressive headlight glare sources. J Cataract Refract Surg. 2019;45(11):1591–1596.
44. Qin Q, Wu Z, Bao L, et al. Evaluation of visual quality after EVO-ICL implantation for hypermyopia: Observační studie. Medicine (Baltimore). 2019;98(44):e17677.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology
2020 Issue 5-
All articles in this issue
- Carotid-cavernous fistula from the perspective of an ophthalmologist A Review
- PRESBYOPIA MANAGEMENT WITH DIFFRACTIVE PHAKIC POSTERIOR CHAMBER IOL
- HIGHLIGHTS OF HYPERTENSIVE AND NORMOTENSIVE GLAUCOMA
- HIGHLIGHTS OF ADVANCES IN MEDICAL RETINA FROM THE VIRTUAL WORLD OPHTHALMOLOGY CONGRESS 2020
- PUPILLOTONIA AND ADIE SYNDROME
- TRANSSCLERAL DIODE CYCLOPHOTOCOAGULATION IN TREATMENT OF GLAUCOMA
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- PUPILLOTONIA AND ADIE SYNDROME
- Carotid-cavernous fistula from the perspective of an ophthalmologist A Review
- TRANSSCLERAL DIODE CYCLOPHOTOCOAGULATION IN TREATMENT OF GLAUCOMA
- HIGHLIGHTS OF HYPERTENSIVE AND NORMOTENSIVE GLAUCOMA
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

