-
Medical journals
- Career
Sentinel Lymph Node in Thin and Thick Melanoma
Authors: L. Roncati 1,2; F. Piscioli 1; T. Pusiol 1
Authors‘ workplace: Provincial Health Care Services, Institute of Pathology, Santa Maria del Carmine Hospital, Rovereto, Italy 1; Department of Diagnostic and Clinical Medicine and of Public Health, Section of Pathology, University of Modena and Reggio Emilia, Modena, Italy 2
Published in: Klin Onkol 2016; 29(5): 393-394
Category: Letter to Editor
The recommendation for sentinel lymph node biopsy (SLNB) is a controversial problem in the management of cutaneous malignant melanoma (CMM). Rovere et al. performed a study with the objective to assess the epidemiologic profile of patients with CMM, who underwent SLNB in Blumenau-Santa Caterina region in Brazil [1]. According to ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of CMM, the authors wrote that SLNB is recommended for CMM with thick - ness > 1 mm. Rovere et al. concluded that Breslow thickness, ulceration, nodular subtype, Clark‘s level IV are associated with SLNB status [1]. We believe that recommendations for SLNB should reflect the biological concept of CMM evolution. The histogenetic theory considers two steps of CMM progression – the radial growth phase (RGP) and vertical growth phase (VGP), with the exception of nodular CMM lacking RGP [2–4]. Two sub-categories of RGP are recognized – the intra-epidermal RGP (in situ melanoma) and the micro-invasive RGP. The former is characterized by proliferation of malignant, transformed melanocytes above the basement membrane (Clark’s level I), with pagetoid or lentiginous morphologic pattern. The latter shows invasion into the papillary dermis (Clark‘s level II or III), with presence of single cells or small nests, in absence of tumor nodule or papule. The dermal nests are invariably smaller than the junctional ones, while the cytological appearance of the junctional and dermal component is overlapping. The absence of dermal mitoses is an absolute criterion; in fact, micro-invasive RGP lacks metastatic potential [2–4]. However, according to our observations, metastases may be found in 1–2% of micro-invasive RGPs, when they are associated with significant regression (> 75%; > 0.75 mm in depth). Therefore, it is likely that this regression may incorporate melanoma cells able to metastasize, with metastases prior the occurrence of regression [5]. VGP designates the point at which CMM becomes biologically capable of producing metastatic events. The tumor infiltrates as nodules of malignant melanocytes, filling the superficial papillary dermis with tendency for deep invasion into reticular dermis (Clark’s le-vel IV) and subcutaneous tissue (Clark’s level V) [2–4]. For definition, VGP includ - es the properties of ‚tumorigenicity‘ and ‚mitogenicity‘ [2–4]. Because of the increasing frequency of thin me-lanomas, there is a great need to develop more refined predictors of SLNB. Today, the scientific community is focusing on the clinical significance of different histological subtypes of thin melanoma [6]. In fact, there is a subset of patients affected by thin melanoma, who develop nodal or distant metastases with worse prognosis [7]. From our experience, as reported in Tab. 1, thin melanoma can be categorized in the following four histological subtypes: I. the intra-epidermal RGP; II. the non - -tumorigenic micro-invasive RGP without regression; III. the micro-invasive RGP with regression of uncertain tumorigenic potential; IV. the tumorigenic early invasive VGP [8]. In micro-invasive RGP with regression and in all cases of VGP, including the early invasive VGP of thin (≤ 1 mm) melanoma (pT1) and the invasive VGP of thick (> 1 mm) melanoma (pT2, pT3, pT4), the SNLB should be performed, independently from other risk factors, because the tumor has the potential to metastasize.
1. The malignant melanocytic lesions of the skin can be subdivided, according to the Breslow thickness, in thin melanoma (≤ 1 mm) or thick melanoma (> 1 mm). 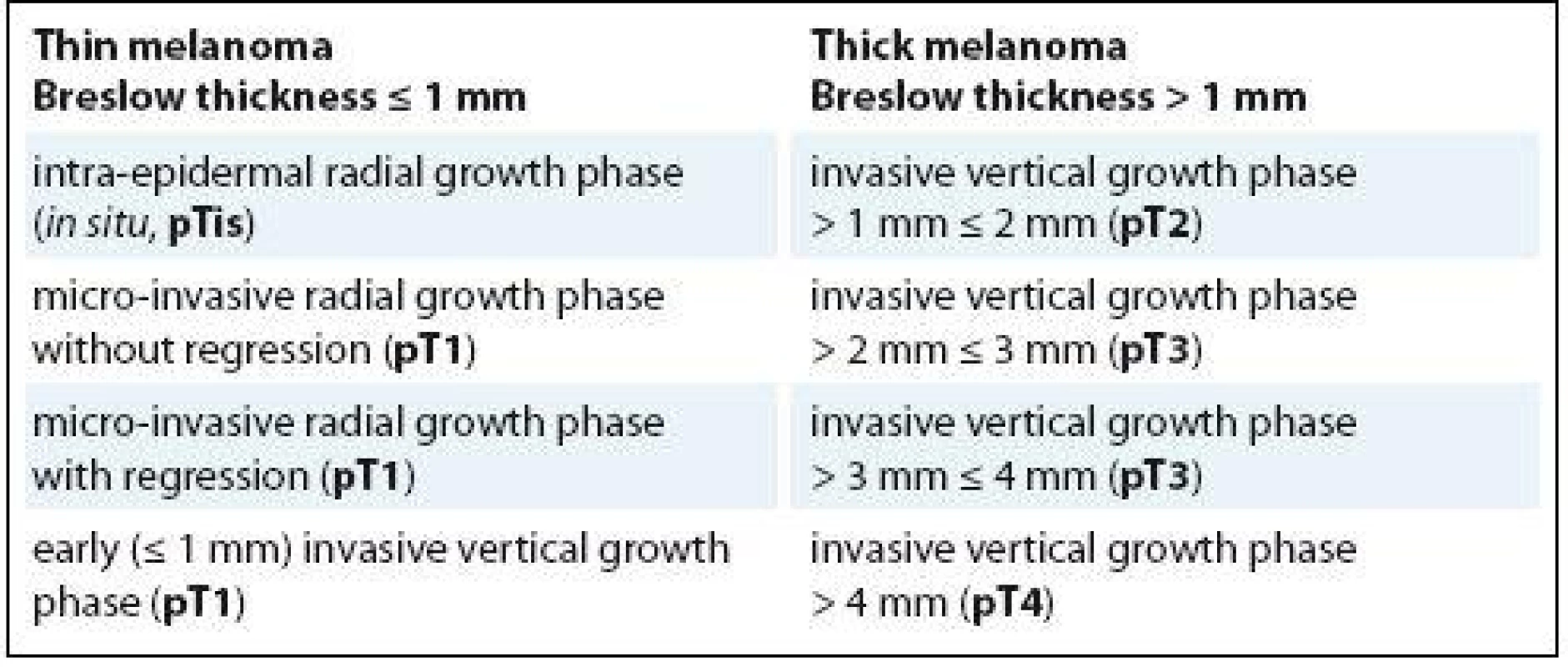
The intra-epidermal radial growth phase and the micro-invasive radial growth phase without regression of thin melanoma are devoid of tumorigenic potential, while the micro-invasive radial growth phase with regression is burdened by an uncertain metastatic potential (at diagnosis). The early invasive vertical growth phase of thin melanoma and the subcategories of invasive vertical growth phase of thick melanoma show all tumorigenic potential, directly correlated to the depth of invasion and mitogenicity (pTis, pT1, pT2, pT3, pT4 and a/b specifi cations are adapted from the AJCC staging system). a and b specifi cations are assigned based on ulceration and number of mitoses for mm2 a – without ulceration and mitoses < 1/mm2 b – with ulceration or mitoses ≥ 1/mm2 Dr. Luca Roncati, MD, PhD
Department of Diagnostic and Clinical Medicine and of Public Health Section of Pathology
University of Modena and Reggio Emilia
Policlinico Hospital I-41124 Modena (MO)
Italy
e-mail: emailmedical@gmail.com
Submitted: 26. 9. 2016
Sources
1. Rovere RK, Silva de Lima A, DeMarchi V et al. Sentinel lymph node in melanoma – a study conducted in the south of Brazil. Klin Onkol 2016; 29 (4): 274–278. doi: 10.14735/amko2016274.
2. Pusiol T, Piscioli F, Speziali L et al. Clinical Features, dermoscopic patterns, and histological diagnostic model for melanocytic tumors of uncertain malignant potential (MELTUMP). Acta Dermatovenerol Croat 2015; 23 (3): 185–194.
3. Piscioli F, Pusiol T, Roncati L. Diagnostic approach to melanocytic lesion of unknown malignant potential. Melanoma Res 2016; 26 (1): 91–92. doi: 10.1097/CMR. 0000000000000215.
4. Piscioli F, Pusiol T, Roncati L. Diagnostic disputes regarding atypical melanocytic lesions can be solved by using the term MELTUMP. Turk Patoloji Derg 2016; 32 (1): 63–64. doi: 10.5146/tjpath.2015.01330.
5. Piscioli F, Pusiol T, Roncati L. Histopathological determination of thin melanomas at risk for metastasis. Melanoma Res. In press 2016. doi: 10.1097/CMR.000 0000000000288.
6. Piscioli F, Pusiol T, Roncati L. Nowadays a histological sub-typing of thin melanoma is demanded for a proper patient management. J Plast Reconstr Aesthet Surg. In press 2016. pii: S1748-6815 (16) 30236-4. doi: 10.1016/j.bjps.2016.08.026.
7. Piscioli F, Pusiol T, Roncati L. Choosing wisely the patients affected by thin melanoma to be submitted for sentinel lymph node biopsy. J Am Acad Dermatol. In press 2016.
8. Piscioli F, Pusiol T, Roncati L. Thin melanoma subtyping fits well with the American Joint Committee on Cancer staging system. Melanoma Res. In press 2016. doi: 10.1097/CMR.0000000000000301.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2016 Issue 5-
All articles in this issue
- The Importance of MITF Signaling Pathway in the Regulation of Proliferation and Invasiveness of Malignant Melanoma
- Expression of ATP-binding Cassette Proteins Pgp, MRP1, and MRP3 in Malignant and Benign Ovarian Lesions
- Multimodal Therapy of Recurrent Malignant Schwannoma
- Hyperthermic Isolated Limb Perfusion Combined with Tasonermin – a Perfusion Leakage Monitoring Technique
- Glycine-N-methyltransferase and Malignant Diseases of the Prostate
- Prognostic and Predictive Factors for Pancreatic Adenocarcinoma
- Treatment of Relapsed and Refractory Hodgkin Lymphoma – Recommendations of the Czech Hodgkin Lymphoma Study Group
- The Impact of High Protein Nutritional Support on Clinical Outcomes and Treatment Costs of Patients with Colorectal Cancer
- Malignant Mesothelioma of the Tunica Vaginalis Testis. A Clinicopathologic Analysis of Two Cases with a Review of the Literature
- Sentinel Lymph Node in Thin and Thick Melanoma
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- The Impact of High Protein Nutritional Support on Clinical Outcomes and Treatment Costs of Patients with Colorectal Cancer
- Treatment of Relapsed and Refractory Hodgkin Lymphoma – Recommendations of the Czech Hodgkin Lymphoma Study Group
- Multimodal Therapy of Recurrent Malignant Schwannoma
- Malignant Mesothelioma of the Tunica Vaginalis Testis. A Clinicopathologic Analysis of Two Cases with a Review of the Literature
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

