-
Medical journals
- Career
TREATMENT OF STAGES III−IV OF THE DUPUYTREN’S DISEASE USING A PERSONAL APPROACH: PERCUTANEOUS NEEDLE FASCIOTOMY (PNF) AND MINIMAL INVASIVE SELECTIVE APONEURECTOMY
Authors: B. Corradino; S. Di Lorenzo; F. Moschella
Authors‘ workplace: Dipartimento di Discipline Chirurgiche e Oncologiche. Sezione di Chirurgia Plastica, Università degli Studi di Palermo, Italy
Published in: ACTA CHIRURGIAE PLASTICAE, 55, 1, 2013, pp. 19-22
INTRODUCTION
The first percutaneous fasciotomy was performed in 1777 by H. Cline, using a small scalpel. It involved sectioning of the pathological Dupuytren’s cords. Gradually, Dupuytren’s Disease surgery became more aggressive (resulting in total palmar fasciotomy) and only recently the percutaneous needle fasciotomy (PNF) regained popularity as a simple and reliable technique particularly suitable for the elderly patients with stage III and IV of Tubiana classification of the Dupuytren’s Disease (Table 1) with prevailing contracture at a MCP and PIP joint level (1).
1. Tubiana’s classification of Dupuytren’s desease 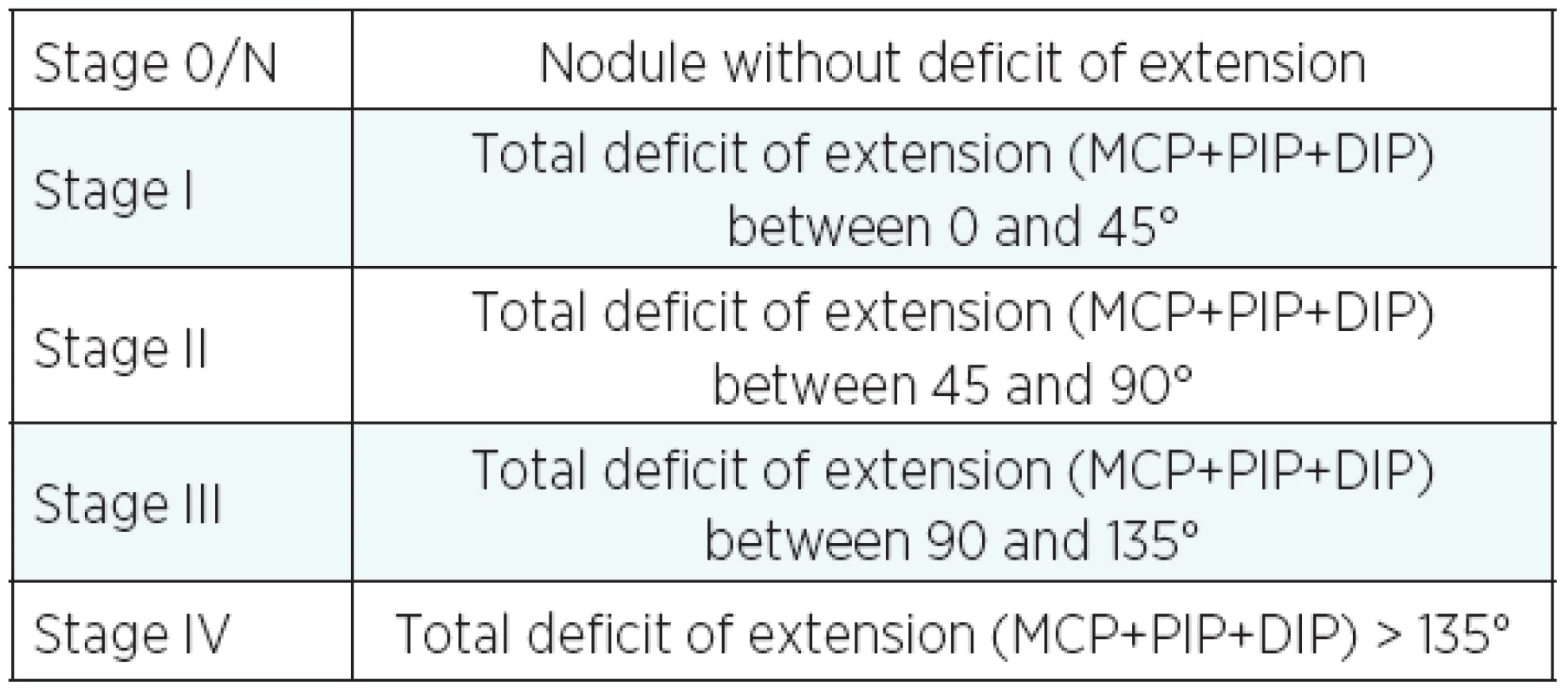
As several studies have shown, the short-term results of the PNF are excellent but unstable over time. The high recurrence rate three years after treatment (8−58%) has limited its use to only a few selected cases (patients with multiple pathologies, the elderly and those with lack of motivation) (2, 3)
The authors present a personal approach to stages III and IV of Dupuytren’s Disease, where a minimal invasive selective aponeurectomy on the previously treated cord is carried out approximatively 40 days after the PNF drastically reducing the recurrence rate after 48 months.
MATERIAL AND METHODS
From January 2007 to February 2012 the same surgeon operated 23 fingers on 20 consecutive patients using this technique with two operations.
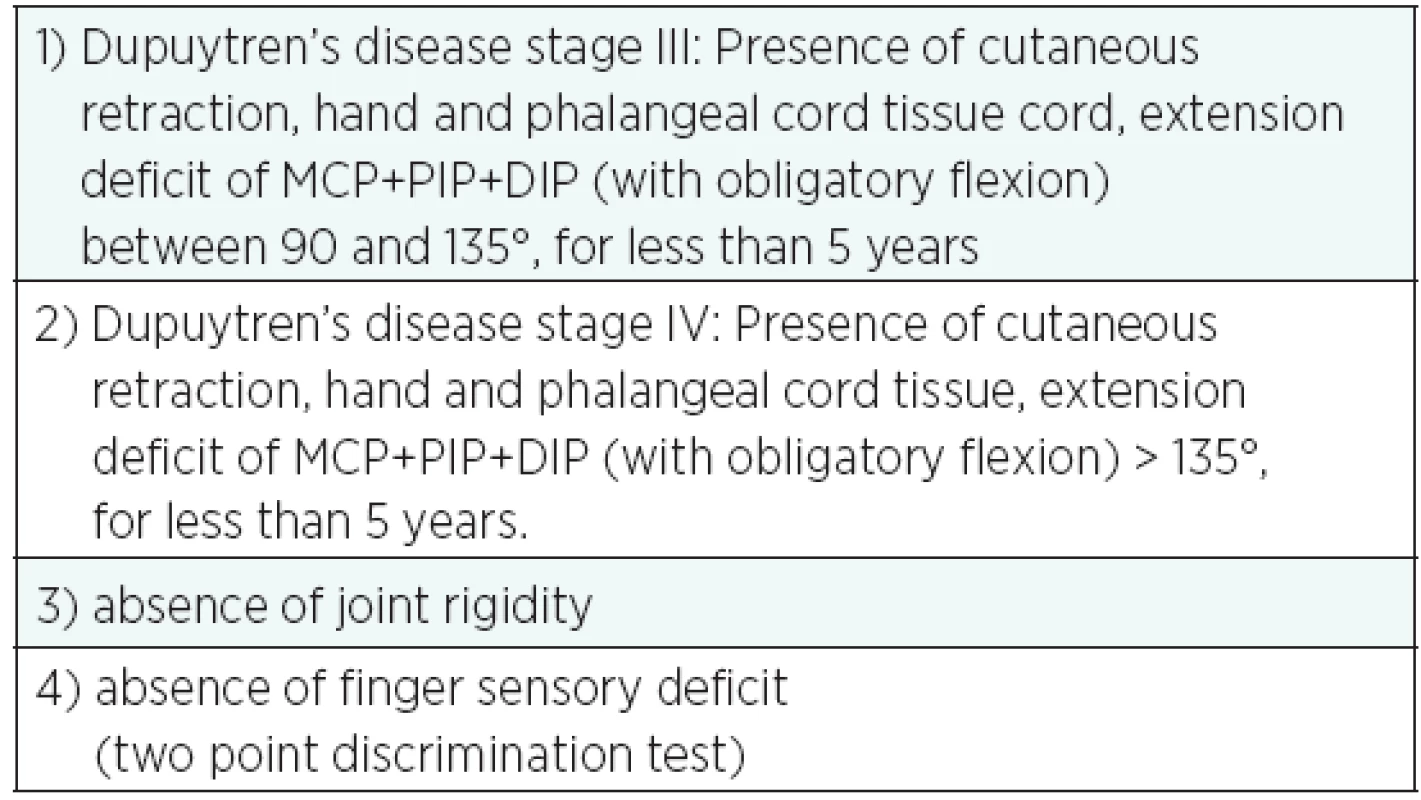
Careful selection of the patients enabled to exclude all cases where the disease had been present for more than 5 years or where there were clear signs of joint rigidity. Signs of osteoarthritis in the joints of the hand are a contraindication to this kind of treatment.
With advanced disease, that is, presence of the disease for more than 5 years, there is a further risk of joint rigidity. That is why patients with such case history are not put forward for treatment.
At the pre-operation stage, each patient’s sensory level on the finger affected by Dupuytren’s Disease is assessed using the two point discrimination test; in fact, some patients show a sensory deficit of the affected finger due to a compression of the finger’s neurovascular bundle by the cord tissue. Identifying a sensory deficit prior to the operation is also important from a medical-legal point of view, because in these cases there is a high risk of iatrogenic lesion of the digital nerve, dislocated from the original site at the PIP level. In these cases, the traditional operation is recommended in order to prevent further damage to the nerve.
14 fingers were at stage III, 9 at stage IV. The operated finger was the ring finger in 60% of cases and little finger in 40% of cases.
The average age of the treated patients was 70, 80% of whom were males.
Patients who met the selection criteria as shown in the table underwent routine examinations and hand x-rays prior to the operation (Table 2).
SURGICAL TECHNIQUES
I. operation
The first operation that is carried out in an outpatient setting under wrist block anaesthesia (block of the ulnar and median nerves at the wrist level using mepivacaine 2% (max dose 6ml) is the percutaneous needle fasciotomy. The average time is 15 minutes.
With the hand in supine position, the cord tissue is identified. In all cases, it was the ring and little finger that were affected by the disease.
The 19G needle penetrates the skin at the distal palm crease in a layer parallel to the skin surface to release acreted skin from the cord tissue underneath firstly in the MCP a PIP area. Then the needle moves back and forth at a 45 degree inclination to the skin surface to interrupt cord tissue. This movement is repeated several times until the band breaks or until division has been achieved. In most cases, it is possible to hear a cracking sound as the cord breaks. The affected finger is passively extended. (Fig. 1, 2, 3a,b) The patient can instantly flex and actively extend the finger. A small compressive dressing is applied for 24 hours to keep the finger extended.
Fig. 1. Pre-op, stage IV. Elderly patient, age 85, that met selection criteria 
Fig. 2. PNF. The 19G needle penetrates firstly parallel to skin in order to release the skin from the cord underneath, then with 45 degree inclination to break the tissue cord with an entry and exit movements until the band breaks 
Fig. 3a,b. Post PNF. The affected finger is passively extended. The patient can instantly flex and actively extend the finger 
Between operations
After this period, the dressing is removed and a static splint with finger in extension is kept for 8 days.
After 8 days, the static splint is removed and the patient is encouraged to start actively flexing and extending his/her finger for about 15 days (active mobilisation). During the night, a splint is used to hold the finger in extension.
30−40 days after the PNF, the patient undergoes the second operation.
II. operation
The second operation, performed in outpatient setting, is again carried out under nerve block anaesthesia. The average time is 15 minutes. No tourniquet is required.
Using 2 small transverse incisions on the distal palm crease and on the PIP, according to McCash, the residual tissue cord is removed in open surgery (Fig. 4). At the end of the surgery, a compressive dressing is positioned and kept for approximately 24 hours.
Fig. 4. Minimal invasive aponeurectomy, 40 days after PNF using 2 incisions 
The patient is encouraged to move the operated finger early. Once the stitches are removed, the early outcome of the operation is satisfactory and a finger’s complete active extension is achieved.
RESULTS
Follow up was carried out for approximately 48 months (min. follow up was 40 months, max. 54), and no relapse after treatment was observed (Fig. 5a,b).
Fig. 5 a,b. Result, no recurrence after 48 months 
In two cases, the correction was incomplete due to osteoarthritis of the PIP joints.
No major complications were observed; no infection, hematoma, arterial or digital nerve injury occurred. Only 3 cases of dehiscence of the sutures caused by skin maceration with signs of inflammation were observed. These were self-limited.
DISCUSSION
The traditional treatment of the Dupuytren’s Disease at an advanced stage (III−IV) involves a particularly invasive operation associated with several localized complications, disadvantages or risk of relapse. If, on the one hand, the selective aponeurectomy using traditional techniques allows for all affected tissues to be radically removed, on the other hand it is a much more invasive procedure and reconstructive surgery is often mandatory.
The adherence of the skin to the fibrous tissue often imposes the sacrifice of the skin itself, requiring a cover with skin grafts or, rarely, local flaps, the failure of which complicates the post-operative course.
With traditional techniques, the surgical time is often longer (often imposing a change in anaesthesia from brachial plexus nerve block to deeply sedating the patient, especially if a surgical treatment for joint rigidity is necessary). The hospitalization and post-operative recovery periods also become longer (3, 4).
The treatment of stages III and IV of the Dupuytren’s Disease using PNF followed 30−40 days later by a minimal invasive selective aponeurectomy of the treated cord will produce satisfactory, long lasting functional results, in carefully selected cases.
The PNF before the mini invasive aponeurectomy allows the skin over the cord to be preserved, without the need to keep the wound on the palm open or to use a skin graft or flaps to repair the tissue loss. As described in literature, the recurrence rate after the PNF is higher without the second operation (minimal invasive selective aponeurectomy) (2, 3).
A careful clinical examination of the patient is necessary for the selection of the patients. The presence of a rigid joint requires the use of traditional techniques, including the treatment of the joint. If there is no extension of the finger after PNF due to undiagnosed joint rigidity, traditional aponeurectomy with open surgical treatment of the joint block is necessary. Only in one case, not included in this study, did the rigidity of the MCP show up after PNF, requiring a more aggressive surgical treatment.
The presence of a sensory deficit prior to the operation is an absolute contraindication to the operation, as this would imply a dislocation of the neurovascular bundle by the cord tissue, with a higher risk of its injury during the percutaneous cordotomy. This is why the two-point discrimination test is performed in every patient. In literature, reported incidence of injury to the finger nerve after PNF is 3%. This particularly high percentage can be attributed to the pathological dislocation of the digital neurovascular bundle in Dupuytren’s Disease. Our experience shows that there were no cases of post-operative sensory deficit attributable to a nerve damage caused by the needle which is most probably a result of the careful selection of the patients who were put forward for this therapeutic strategy (2).
The advantages of PNF+ miniSA (selective aponeurectomy) can be summarized as follows:
- they are quick operations (approximately 15 minutes each) performed in an outpatient setting that do not require hospitalization;
- debilitated patients or those with systemic multiple pathologies can safely be put forward for this therapeutic strategy;
- since no retracted skin is sacrificed, there is no need for restoration of skin cover using grafts or flaps;
- all of the patients experience a quick functional recovery and rapid return to normal life;
- both operations are low cost;
- during an average follow-up of 48 months, the rate of recurrence was lower compared to PNF alone (as high as 58%).
Disadvantages are to be found in the double surgical, anaesthesiological and rehabilitative time. It is also possible that the nerve structure, or more rarely, tendons could become damaged should there be a failure to observe inclusion/exclusion criteria.
Our early experience suggests that percutaneous needle fasciotomy followed by a minimal selective aponeurectomy of the pathological cord, using a small skin incision, is an easy technique and simple procedure that could be the gold standard treatment for selected patients with stage III and IV of Dupuytren’s disease, but further investigations, more cases and a longer follow-up allow us to obtain more statistically significant data.
The authors declare that they have no conflicts of interest to disclose.
Address for correspondence:
Bartolo Corradino, MD.,
Dipartimento di Discipline Chirurgiche
e Oncologiche. Sezione di Chirurgia Plastica Università degli Studi di Palermo
Via del vespro n. 129
Palermo
Italy
E-mail: bartolocorradino@unipa.it, dilsister@libero.it
Sources
1. Van Rijssen A.L.,Werker P.M.N. Percutaneous needle fasciotomy in Dupuytren‘s disease. J. Hand Surg. Br., 31(5), 2006, p. 498−501.
2. Foucher G., Medina J., Navarro R. Percutaneous needle aponeurotomy: complications and results. J. Hand Surg. Br., 28(5), 2003, p. 427−431.
3. Van Rijessen A.L., Gerberandy F.S.J., Linden H.T, Klip H., Werker P. M.N. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: a 6-week follow-up study. J. Hand Surg. Br., 31(5), 2006, p. 717−725.
4. Cordova A., Tripoli M., Corradino B., Napoli P., Moschella F. Dupuytren’s contracture: an update of biomolecular aspects and therapeutic perspectives. J. Hand Surg. Br., 30(6), 2005, p. 557−562.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2013 Issue 1-
All articles in this issue
- CONGENITAL HAND DEFORMITIES – A CLINICAL REPORT OF 191 PATIENTS
- “DOWNWARD STEPS TECHNIQUE” WITH CO2 ULTRAPULSED LASER FOR THE TREATMENT OF RHINOPHYMA: OUR PROTOCOL
- TREATMENT OF STAGES III−IV OF THE DUPUYTREN’S DISEASE USING A PERSONAL APPROACH: PERCUTANEOUS NEEDLE FASCIOTOMY (PNF) AND MINIMAL INVASIVE SELECTIVE APONEURECTOMY
- COMBINED TRIGGERING AT THE WRIST AND FINGERS AND SEVERE CARPAL TUNNEL SYNDROME CAUSED BY MACRODYSTROPHIA LIPOMATOSA. CASE REPORT AND REVIEW OF LITERATURE
- CHANGES IN DONOR SITE SELECTION IN LOWER LIMB FREE FLAP RECONSTRUCTIONS BY INTEGRATING DUPLEX ULTRASONOGRAPHY IN THE PREOPERATIVE DESIGN
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- TREATMENT OF STAGES III−IV OF THE DUPUYTREN’S DISEASE USING A PERSONAL APPROACH: PERCUTANEOUS NEEDLE FASCIOTOMY (PNF) AND MINIMAL INVASIVE SELECTIVE APONEURECTOMY
- CONGENITAL HAND DEFORMITIES – A CLINICAL REPORT OF 191 PATIENTS
- “DOWNWARD STEPS TECHNIQUE” WITH CO2 ULTRAPULSED LASER FOR THE TREATMENT OF RHINOPHYMA: OUR PROTOCOL
- COMBINED TRIGGERING AT THE WRIST AND FINGERS AND SEVERE CARPAL TUNNEL SYNDROME CAUSED BY MACRODYSTROPHIA LIPOMATOSA. CASE REPORT AND REVIEW OF LITERATURE
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career