-
Medical journals
- Career
The comparison of effectivity in breast cancer prevention between skin sparing and subcutaneous mastectomy – 20 years of experience
Authors: A. Berkeš; L. Streit; L. Dražan; J. Veselý; A. Bajus; T. Kubek; O. Šedivý; K. Kanuščák; K. Feiková; O. Strmiska; M. Bohušová
Authors‘ workplace: Department of Plastic and Aesthetic Surgery, Faculty of Medicine, Masaryk University and St. Anne’s University Hospital, Brno, Czech Republic
Published in: ACTA CHIRURGIAE PLASTICAE, 65, 3-4, 2023, pp. 112-116
doi: https://doi.org/10.48095/ccachp2023112Introduction
Breast cancer is the most common diagnosed cancer among women worldwide, with the steady increase of incidence rate albeit the decrease in mortality in all age groups. Overall, it accounts for more than 1 in 10 newly diagnosed cancers and, following lung cancer, it is the second most common cause of death among females related to cancer [1]. Breast cancer places an enormous burden both on the healthcare systems as well as patients worldwide. The factor that has been strongly associated with the medicine in 21st century is the quality of life, which, according to the World Health Organization, is person’s perception and satisfaction with life and their general appraisal of their level of functional well-being [2]. The patients who are diagnosed with breast cancer are anxious about their future, worry about pain, diminishing social relationships and experience stress and depression [3]. As these symptoms increase, the quality of life goes down. However, patients who successfully made the transition from cancer patient to cancer survivor are able to overcome those issues and their quality of life improves [4].
Having taken the aforementioned facts into account, there is little to no surprise that several prophylactic measures have been implemented into healthcare systems to increase chances of either cancer prevention or diagnosing the cancer at early stage. The most common procedure implemented almost in every country as the breast cancer screening is mammography which allows for the early diagnosis. This method is effective and reduces the number of advanced and fatal breast cancers [5].
To reduce the overall number of breast cancer incidence rate, the prophylactic mastectomy strategy, which is the removal of the presumably healthy breast for cancer prevention, was developed. It gained popularity among non-medical professionals, following the course of treatment of Angelina Jolie, who underwent the bilateral prophylactic mastectomy due to the positivity of BRCA1 mutation, acquiring the name Angelina Jolie effect [6]. The patients who are subject to this procedure are at high-risk of developing breast cancer. The most common indication in the genetic subgroup of patients is the presence of the BRCA1 and BRCA2 mutation, which is found in 20% of family breast cancer clusters [7]. Other mutations found in these patients include p53 and PTEN (phosphatase and tensin homolog) mutations, both of which increase the risk of developing breast cancer throughout lifetime around 25% [8,9]. However, even in the lack of the proven point mutation, family history alone is sufficient to place the patient into high-risk category and therefore be subjected to the prophylactic mastectomy [10].
The prophylactic mastectomy can be performed in different manners, differing in the surgical approach to the breast structures. The simplest approach being so called total or simple mastectomy, where both nipple areola complex as well as breast tissue are removed via elliptical incision [11]. The next step in development of surgical technique is the skin sparing mastectomy (SSM), where the nipple areola complex is removed via periareolar incision, while preserving the natural skin envelope of the breast [12]. An extension of this particular technique is called nipple sparing mastectomy or subcutaneous mastectomy (SCM) where the nipple areola complex is preserved [13]. The in between and less frequently used technique is the areola sparing, while the SSM was found to be of same oncological radicality as the modified radical mastectomy by a meta-analysis by Lanitis et al. [14]. The SCM, due to the fact of the gland left behind the nipple-areola complex, may be of lower oncological efficacy [15]. However, although potentially less radical in terms of breast cancer prevention, the study by de la Pena-Salcedo with the 25 years follow-up proves safety of SCM with reduction in breast cancer incidence of 95% [16].
In this paper, we want to evaluate the institutional long-term experiences with prophylactic mastectomy and to compare efficacy of SCM vs. SSM on the breast cancer prevention in high-risk patients. All of this along with the analysis of methods used for the breast reconstruction in these patients.
Methods
Two hundred and one patient who underwent bilateral SCM, SSM or areola sparing mastectomies (ASM) from January 2002 to December 2019 were included in a retrospective cohort study. There were 11 patients excluded from this study, 10 patients due the lack of data, and 1 patient underwent primarily prophylactic mastectomy in different institution and there was secondary mastectomy performed at our department. The patients included in the study were at high-risk to develop breast cancer either through positive genetic mutations or positive family history of breast cancer. All the patients also underwent a bilateral breast reconstruction either with an implant, tissue expander, lipofilling or with an abdominal flap.
The patients were divided into three study groups based on the type of prophylactic mastectomy: SCM, SSM, ASM. The clinical data we were collecting included age, weight, height, BMI, method of reconstruction, incidence rate of breast cancers among included patients, incidence of genetic mutation (BRCA1/2 or other).
The information on possible occurrence of cancer was collected via questionnaire by physical mail and telephone consultation. The obtained data were compared with the data of the National Oncologic Database.
The continuous dependent variables were evaluated with the Kruskal-Wallis for the continuous dependent variables. The normality was tested with the Shapiro-Wilk test. Subsequently, Dunn’s test was chosen as the post-hoc test. The data are presented as median and interquartile range (IQR). To test the occurrence of breast cancers and difference among groups, the Fisher test was used with the subsequent correction using the Bonferroni correction. All the statistical analysis were performed with the R program version 4.2.2.
Results
The median age of patients was 40.0 years with the median BMI of 24.8. Of all 201 patients, 90 patients underwent SSM, 102 SCM and 9 were treated with ASM. Patients who underwent SSM are statistically significantly older, with higher body mass and BMI than those who underwent SCM. Additionally, SSM group had significantly higher BMI than those who underwent ASM (Tab. 1).
In our study, the most common type of reconstructions following prophylactic mastectomy are abdominal flap and implant-based reconstruction. One patient underwent both types of these methods for breast reconstruction. Three patients did not have their breast reconstructed. While reconstructing the breast, the flaps used for right breast were DIEP/DIEA and TRAM for 78 (90.7%) and 8 (9.3%) patients, respectively. While the numbers for the left breast were 80 (92.0%) and 7 (8.0%), respectively. All clinical data regarding the type of reconstruction are aggregated in Tab. 2.
One hundred and eighty-nine patients (94.0%) of our study group were tested for genetic mutations – 5 of them (2.7%) were negative for all tested mutations, 173 (91.5%) were BRCA1 or BRCA2 positive, while 11 (5.8%) tested positive for other genetic mutations (Tab. 3). In the SCM group, there were 86 (84.3%) BRCA1 or BRCA2 positive, while in the SSM group there were 79 (87.8%) BRCA1 or BRCA2 positive. Eight (88.9%) patients who underwent ASM were positive for BRCA1 or BRCA2 testing. We have found no statistically significant correlation between the presence of genetic mutation and age, weight, height or BMI.
The median follow-up for all groups is 5 years. There was no statistically significant difference between patients undergoing different mastectomy types.
In our study group of 201 patients who underwent preventive mastectomy, there were two cases of breast cancers following surgery. One in the SCM and one in the SSM group. For the SSM patient the tumor was in the left breast, she had a positive family history, and tested positive for BRCA2 mutation. The tumor was diagnosed in the specimen collected from the SSM procedure. She was later diagnosed twice with the breast cancer recurrence in 4 and 6 years following SSM. The patient in the SCM group had a positive family history and tested positive for BRCA1 mutation. She was diagnosed with breast cancer in 2.4 years following the SCM. Due to the limited number of breast cancer occurrence, we were not able to find statistically significant difference between the mastectomy type and the incidence rate of breast cancers.
1. Correlation between clinical data and type of reconstruction. 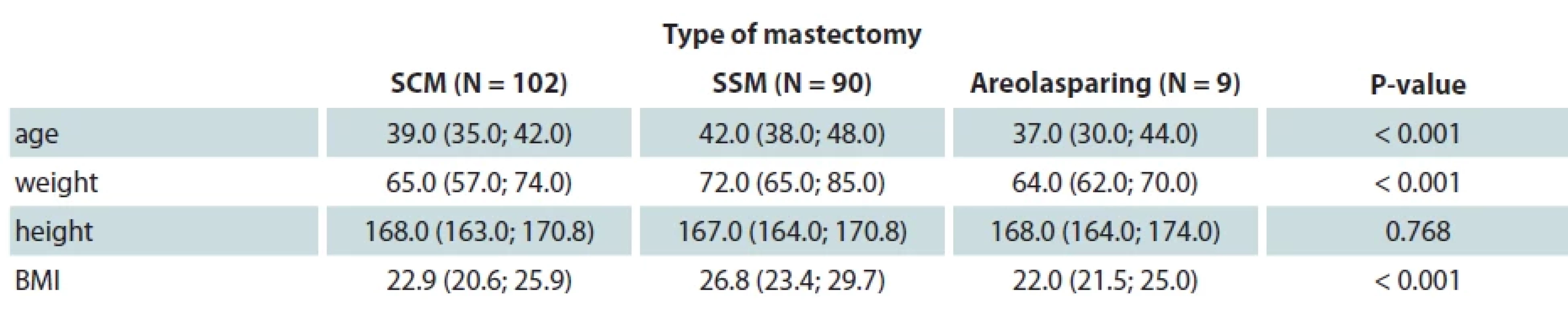
SCM – subcutaneous mastectomy, SSM – skin sparing mastectomy, BMI – body mass index 2. Types of breast reconstruction among patients included in our study. 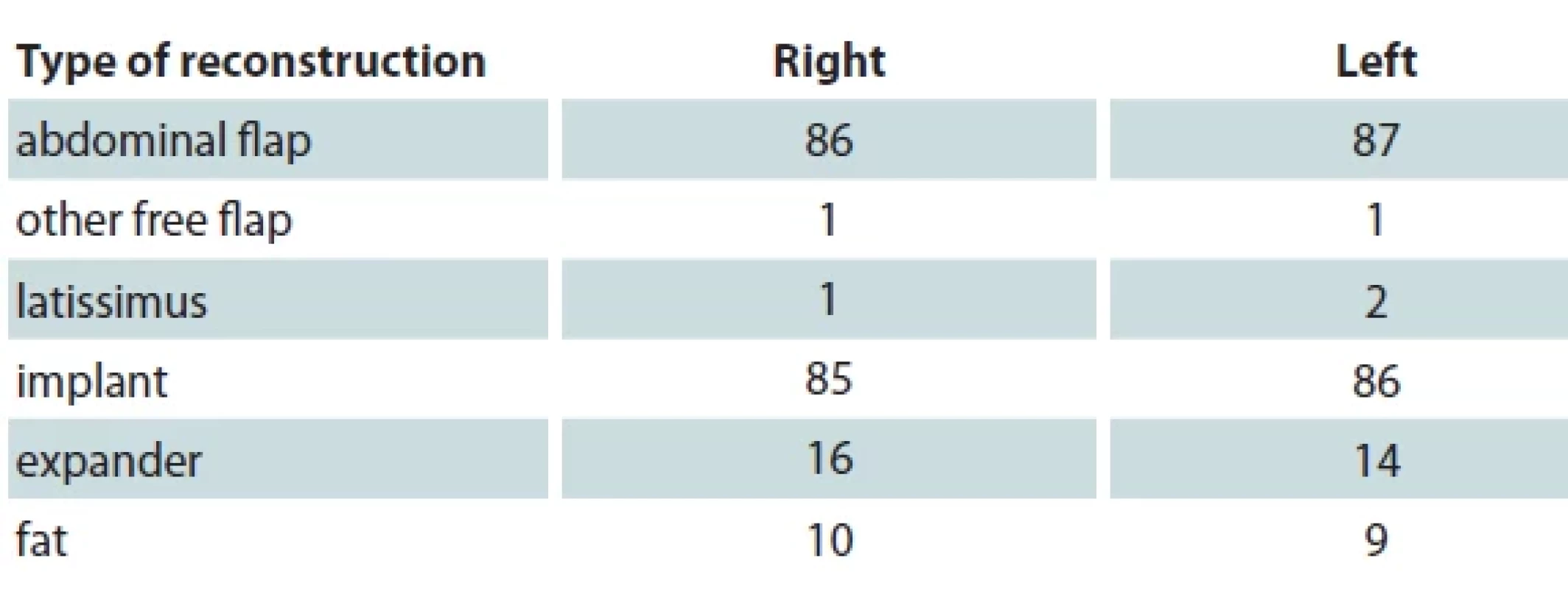
3. Genetic mutation and clinical data. 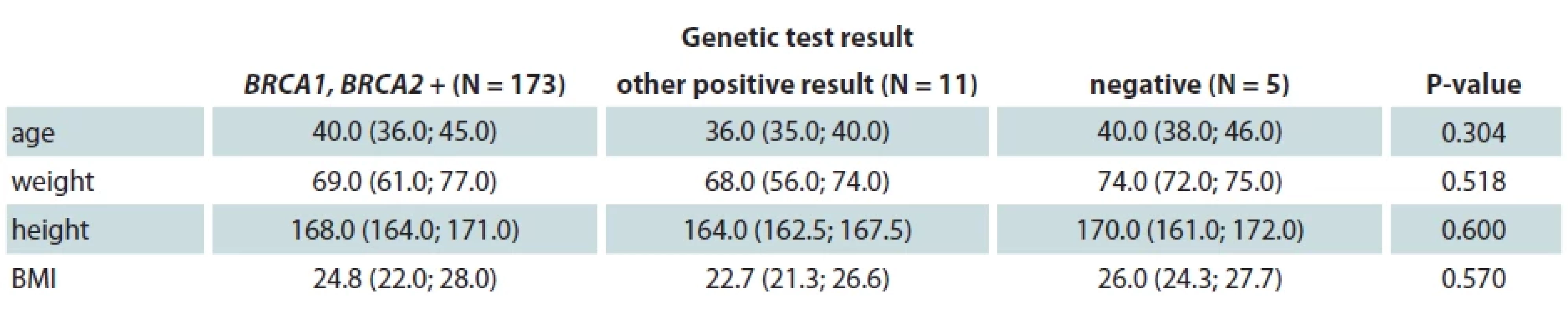
BRCA – breast cancer gene, BMI – body mass index Discussion
The mastectomy for the prevention of the breast cancer remains a subject of the vicious debate. On the one hand, it is the most effective treatment in terms of reducing the risk of breast cancer occurrence and therefore reducing the death rates from any cause between high-risk patients [17]. On the other hand, given the fact that modern screening programs along with better and more sensitive diagnostic tools make it possible, the detection of early breast cancer patients is reaching as high as 97.3% when combining mammography and ultrasound [18,19].
Therefore, proper indication in high-risk patients is crucial in the breast cancer safe and effective prevention process. In our study group, the median age of undergoing all types of mastectomies was 40 years. This age in our opinion is the right moment for preventive operation in high-risk patients because the median age of onset of breast cancer for various types of BRCA1/2 mutation revolves around the age of 44 years [20]. This allows for the proper prevention of the breast cancer occurrence. In our study group, the patients who underwent SSM were of older age and higher BMI than SCM or ASM group. This data is in line of those found in the literature. As the higher mass of the breast is correlated with the BMI, however not with age [21], it is suggested by Wang et al. [22] that larger breasts are considered a relative contraindication for ASL or SCM. This is further reinforced by Tousimis et al. [23] who underlines the importance of low BMI, small breast and absence of ptosis for the safe ASM procedure, while presenting several operative techniques which are able to broaden the indications for this operation.
The main difference between the SCM and SSM is the amount of breast tissue left behind which may potentially cause the discrepancies in the long-term follow-up for the breast cancer incidence rate. Even though several advancements have been achieved in the recent years, the guidelines still recommend leaving glandular tissue as the scaffold for vascularity [24]. Additionally, the problem with tumors located in the central part of the breast is that they are considered to be of more aggressive nature, thus having pooper prognosis than those located in the peripheral parts of the breast [25]. The data shows that the ASM, when performed correctly, has no disadvantage over the traditional mastectomy [26]. In our study, the same principles hold true. Although limited in number, we have not found a statistically significant difference between the SCM and SSM in the incidence rate of breast cancers among the patients who underwent these procedures. However, further studies with longer follow-up are needed to fully support this data.
The patients who were subjected to the prophylactic mastectomies in our department were most often reconstructed with the abdominal flaps and implants. Worldwide, the implant-based reconstructions are the most commonly performed procedures while offering several advantages such as shorter recovery time, shorter time of the reconstruction itself and no donor site morbidity. Additionally, one may use this reconstructive option when operating on a thin patient who may lack the tissue for the flap. On the other hand, the implant-based reconstruction poses several risks. The infection of the implant and occurrence of breast implant associated anaplastic large cell lymphoma (BIA-ALCL). Other risk includes capsular contracture, malposition of the implant and damage to the implant itself resulting in its rupture or exposure. The limiting factor for this type of reconstruction is radiotherapy. When performed in the single stage, the treatment plan may not include radiotherapy as it may damage the implant. On the other hand, when operating on the irradiated breasts, the quality of skin flap may not be sufficient and may require the use of acellular dermal matrix to support the weight bearing skin [27–29].
The abdominal flap reconstruction, on the other hand, allows the patient to restore the breast with the similar tissue which is the paradigm of reconstructive surgery stated by Gilles as replace like with like. This kind of reconstruction not only provides the esthetically pleasing breast but also bypasses the limitations in the implant-based reconstruction. Since the neobreast is formed with all the adjacent tissues, the recipient site does not have to meet the requirements of those in implant-based reconstruction. However, the flap surgery is far more complex and technically challenging, requiring the surgeon to fully comprehend the importance of every step. Due to the presence of several critical phases including the harvesting of the flap, revascularization and shaping of the flap, the procedure is far more time consuming, which limits the patients from the anesthesia point of view.
In our study group, 91.5% of patients who underwent the genetic testing were found to be carriers of either BRCA1 or BRCA2 mutations. This cluster is a well-known risk factor for developing breast cancer [30]. The cumulative risk of developing breast cancer in these patients is close to 70% in the long-term observation [31].
In our study group, two patients developed breast cancer following mastectomy. One was treated with SCM while the other with SSM. The patient who underwent SSM was positive for BRCA1 and patient with SCM had BRCA2 mutation. The incidence rate of breast cancer following prophylactic (or risk reducing?) mastectomy among our study group amounted to 0.99%. This finding is in line with those found in the literature where the match control group with no prophylactic mastectomy experienced almost 25-fold increased rates of breast cancer occurrence [32]. Albeit at the longer mean follow-up of 6.4 years vs. median 5 years follow-up in our study. Of note is that for the SSM close to 50% of patients have some residual breast tissue in the flap, whereas for the SCM the numbers are higher [33].
Conclusion
Prophylactic mastectomy was confirmed to be a reliable strategy for significantly reducing the number of breast cancer incidence in high-risk patients in our study. It was proven effective regardless of the type of mastectomy as there was no difference in the efficacy of prophylactic mastectomies between groups of SCM and SSM. However, due to the limited number of patients, further studies e. g. meta-analysis are needed to achieve higher level of evidence.
Roles of authors
conceptualization – A. Berkeš and L. Streit;
methodology – L. Streit and L. Dražan;
software – K. Kanuščák, K. Feiková, M. Bohušová and O. Šedivý;
validation – L. Streit;
formal analysis – L. Streit., K. Kanuščák, K. Feiková and M. Bohušová;
investigation – A. Berkeš and L. Streit;
resources – T. Kubek and A. Bajus;
data curation – M. Bohušová;
writing original draft preparation – A. Berkeš, L. Streit and L. Dražan;
visualization – K. Feiková, K. Kanuščák and M. Bohušová;
supervision – L. Streit and J. Veselý;
project administration – L. Streit.
All authors have read and agreed to the published version of the manuscript.
Disclosure: The authors have no conflicts of interest to disclose. This study was written with the support of the Specific University Research MUNI/A/1360/2022 and was approved by the institutional ethics committee of St. Anne’s University Hospital (approval number: EK-FNUSA-15/2023) and was conducted according to the tenets of the Helsinki Declaration.
Sources
1. Giaquinto AN., Sung H., Miller KD., et al. Breast cancer statistics 2022. CA Cancer J Clin. 2022, 72 (6): 524–541.
2. World Health Organization. WHOQOL – measuring quality of life. [online]. Available from: https: //www.who.int/tools/whoqol#: ~: text=WHO%20defines%20Quality%20of%20Life,%2C%20expectations%2C%20standards%20and%20concerns.
3. Kwan ML., Ergas IJ., Somkin CP., et al. Quality of life among women recently diagnosed with invasive breast cancer: the pathways study. Breast Cancer Res Treat. 2010, 123 (2): 507–524.
4. Park JH., Jung YS., Kim JY., et al. Determinants of quality of life in women immediately following the completion of primary treatment of breast cancer: a cross-sectional study. PLoS One. 2021, 16 (10): e0258447.
5. Duffy SW., Tabár L., Yen AM., et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020, 126 (13): 2971–2979.
6. Kluger J., Park A. The Angelina effect. Time. 2013, 181 (20): 28–33.
7. Casaubon JT., Kashyap S., Regan JP. BRCA1 and BRCA2 mutations. In: StatPearls [online]. Treasure Island (FL): StatPearls Publishing; 2023.
8. Ochs-Balcom HM., Marian C., Nie J., et al. Adiposity is associated with P53 gene mutations in breast cancer. Breast Cancer Res Treat. 2015, 153 (3): 635–645.
10. Ginsburg O., Bray F., Coleman MP., et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017, 389 (10071): 847–860.
11. Horton CE., Dascombe WH. Total mastectomy: indications and techniques. Clin Plast Surg. 1988, 15 (4): 677–87.
12. Cunnick GH., Mokbel K. Skin-sparing mastectomy. Am J Surg. 2004, 188 (1): 78–84.
13. Hinton CP., Doyle PJ., Blamey RW., et al. Subcutaneous mastectomy for primary operable breast cancer. Br J Surg. 1984, 71 (6): 469–472.
14. Lanitis S., Tekkis PP., Sgourakis G., et al. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer. Ann Surg. 2010, 251 (4): 632–639.
15. Goldman L., Goldwyn R. Some anatomical considerations of subcutaneous mastectomy. Plast Reconstr Surg. 1973, 51 (5): 501–505.
16. de la Peña-Salcedo JA., Soto-Miranda MA., Lopez-Salguero JF. Prophylactic mastectomy: is it worth it? Aesthetic Plast Surg. 2011, 36 (1): 140–148.
17. Lostumbo L., Carbine NE., Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010, 11: CD002748.
18. Coleman C. Early detection and screening for breast cancer. Semin Oncol Nurs. 2017, 33 (2): 141–155.
19. Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology. 2020, 295 (1): 52–53.
20. Okano M., Nomizu T., Tachibana K., et al. The relationship between BRCA-associated breast cancer and age factors: an analysis of the japanese HBOC consortium database. J Hum Genet. 2020, 66 (3): 307–314.
21. Coltman CE., Steele JR., McGhee DE. Breast volume is affected by body mass index but not age. Ergonomics. 2017, 60 (11): 1576–1585.
22. Wang F., Alvarado M., Ewing C., et al. The impact of breast mass on outcomes of total skin-sparing mastectomy and immediate tissue expander–based breast reconstruction. Plast Reconstr Surg. 2015, 135 (3): 672–679.
23. Tousimis E., Haslinger M. Overview of indications for nipple sparing mastectomy. Gland Surg. 2018, 7 (3): 288–300.
24. Sacchini V., Pinotti JA., Barros AC., et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006, 203 : 704–714.
25. Vila J., Gandini S., Gentilini O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast. 2015, 24 (3): 175–181.
26. Wu ZY., Kim HJ., Lee JW., et al. Oncologic out-comes of nipple-sparing mastectomy and immediate reconstruction after neoadjuvant chemotherapy for breast cancer. Ann Surg. 2021, 274 (6): e1196–e1201.
27. Piper ML., Rios-Diaz AJ., Kimia R., et al. Direct-to-implant versus 2-stage breast reconstruction. Ann Plast Surg. 2022, 89 (2): 159–165.
28. Jeon HB., Lee M., Roh TS., et al. Complications including capsular contracture in direct-to-implant breast reconstruction with textured anatomical versus smooth round implants: a single center retrospective analysis. J Breast Cancer. 2023, 26 (1): 25.
29. Margulies IG., Salzberg CA. The use of acellular dermal matrix in breast reconstruction: evolution of techniques over 2 decades. Gland Surg. 2019, 8 (1): 3–10.
30. Flinter F. Medical genetics: advances in brief: estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. J Medical Genetics. 1996, 33 (3): 260.
31. Kuchenbaecker KB., Hopper JL., Barnes DR., et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017, 317 (23): 2402–2416.
32. Rebbeck TR., Friebel T., Lynch HT., et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The Prose Study Group. J Clin Oncol 2004, 22 (6): 1055–1062.
33. Cao D., Tsangaris TN., Kouprina N., et al. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol. 2008, 15 (5): 1330–1340.
Libor Streit, MD, PhD
Department of Plastic and Aesthetic Surgery
Faculty of Medicine, Masaryk University
Berkova 34
612 00 Brno
Czech Republic
e-mail: libor.streit@med.muni.czSubmitted: 22. 5. 2023
Accepted: 4. 1. 2024Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2023 Issue 3-4-
All articles in this issue
- Editorial
- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Scalp arteriovenous malformations – 20 years of experience in a tertiary healthcare centre
- The comparison of effectivity in breast cancer prevention between skin sparing and subcutaneous mastectomy – 20 years of experience
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- A primary cutaneous carcinosarcoma of the retro auricular region, how to treat and literature review
- Combination of cable ties and barbed sutures for fasciotomy closure – two case reports
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
- Abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with subcostal scars – case report
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

