-
Medical journals
- Career
Abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with subcostal scars – case report
Authors: H. F. Mayer; R. M. Palacios Huatuco; T. Ruff A; H. A. Aguilar
Authors‘ workplace: Department of Plastic Surgery, Hospital Italiano de Buenos Aires, University of Buenos Aires Medical School, Hospital Italiano de Buenos Aires University Institute, Buenos Aires, Argentina
Published in: ACTA CHIRURGIAE PLASTICAE, 65, 3-4, 2023, pp. 155-159
doi: https://doi.org/10.48095/ccachp2023155Introduction
Abdominal wall defects encompass a broad spectrum of musculo-fasciocutaneous abnormalities [1]. These defects are predominantly acquired as a result of trauma, previous surgery, infection, and tumour resection [2]. In addition, they can be classified as partial-thickness defects, involving skin and subcutaneous tissue, or full-thickness defects, involving loss of skin, subcutaneous tissue, and musculofascial tissue [1]. To preserve the vascular supply and avoid soft tissue necrosis, previous abdominal scars should always be considered when planning an abdominoplasty.
We present a case of abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with previous scars in the upper abdomen.
Case presentation
A 42-year-old woman with a history of multimorbidity (arterial hypertension, diabetes mellitus, asthma), grade II obesity (BMI 36 kg/m2), smoking, and bilateral subcostal scars from a complicated cholecystectomy. The patient underwent repair of an incisional ventral hernia and a transverse abdominoplasty performed by a senior general surgeon from another institution. However, she evolved with extensive necrosis of the cutaneous-fat panniculus between the bilateral subcostal incision and the abdominoplasty incision, for which she consulted our Plastic Surgery Department.
On physical examination, there was soft tissue loss of vitality and necrotic plaques in a medial area of 50 × 60 cm (Fig. 1). Three sessions of tangential escharectomy were performed to remove devitalized tissue and postoperative open wound care consisted of hydrocolloid dressings and alginate dressings. We achieved a retracted final defect with a healthy granulation bed of 45 × 40 cm.
1. 
A) Necrosis of the abdominal fl ap is observed in the midline of the abdomen from the cranial incision to the caudal incision (dashed yellow lines) and adjacent areas of dehiscence (solid red lines). B) Loss of skin coverage and compromise of subcutaneous tissue. Abdominal wall reconstruction with tissue expanders (TE) was planned with the goal of creating adjacent skin flaps for primary closure. Under general anaesthesia and prior infiltration with a solution containing 2% xylocaine and 1/100,000 diluted adrenaline, suprafascial pockets were made through the margins of the defect and three MENTOR® smooth TEs (Irvine, CA, USA) were implanted. Two 300cc crescent-shaped TEs were placed in the right iliac fossa and left flank. A third 250cc rectangular expander was placed in the right hypochondrium.
During 8 weeks, the TEs were progressively insufflated with volumes ranging from 50 to 150cc of saline, depending on the patient’s tolerance (Fig. 2).
In a second stage, also under general anaesthesia, the TEs were extracted, while preserving the newly formed capsular tissue to reinforce the defect closure with the advancement of dermo-fatty flaps (Fig. 3).
After 5 years of follow-up, the patient achieved satisfactory functional and cosmetic results with long-term stability of the abdominal wall (Fig. 4).
2. 
Patient in standing position. A) Soft tissue defect in the abdominal wall with an area of 1,800 cm2 (45 cm high × 40 cm wide) 2 months after presentation to our department. B) Post-expansion of the three tissue devices, one rectangular expander (dashed yellow lines) and two crescent expanders (dashed red lines). 3. 
Perioperative images. A) Preoperative marking. Approach incisions (solid black lines), location of the neoumbilicus (dashed black circle), and Burow's triangles (dashed triangles). B) Defi nitive defect closure. 4. Postoperative control at 5 years. A) Right oblique view; B) frontal view; C) left oblique view. 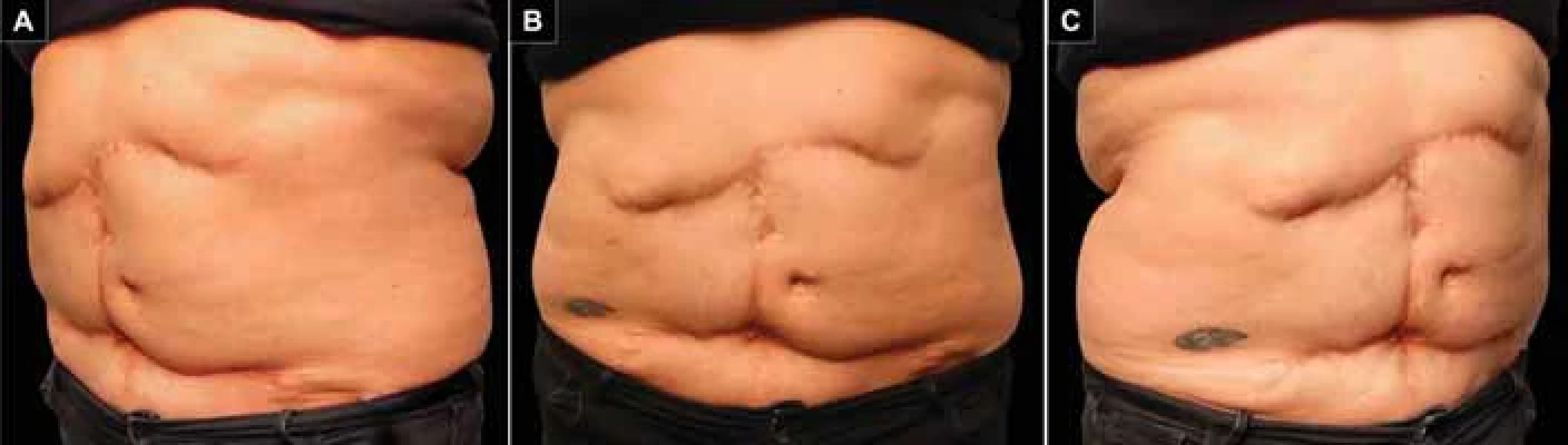
Discussion
Abdominoplasty significantly alters the vascular supply to a significant portion of the abdomen. In a review of 150 patients who underwent abdominoplasty, 72% had a previous abdominal scar. Supraumbilical scars were associated with a higher rate of postoperative complications [3]. In particular, supraumbilical or subcostal transverse scars compromise the blood supply of the superior epigastric arteries [4]. Therefore, these patients are at increased risk of abdominal flap necrosis between incisions. This report highlights the importance of adequate assessment of the abdominal wall with previous scars before an abdominoplasty.
In our patient, the defect was a complication after an incisional hernioplasty with transverse abdominoplasty performed by a general surgeon in a patient with a bilateral subcostal incision. A multidisciplinary approach including plastic surgery should be mandatory to avoid this type of complication. However, no surgical specialty is free from complications. There have been reports of cases of abdominal wall necrosis following body contouring procedures performed by plastic surgeons, especially when using power-assisted liposuction devices [5]. Although training in plastic surgery does not guarantee success, plastic surgeons have the armamentarium and knowledge to reconstruct extensive soft tissue defects such as this.
Abdominal wall defects with extensive soft tissue loss represent a reconstructive challenge. In general, there are two reconstructive strategies when primary closure is not possible. The first option is to approximate the tissue using relaxing incisions or preoperative measures such as tissue expansion or progressive pneumoperitoneum [6]. The second option is to cover the defect with autologous tissue with or without synthetic products [7]. In addition, vacuum-assisted systems can be useful tools for wound temporization, reducing edema, bacterial colonization, and increasing blood flow. However, our patient did not agree to use this device.
Our reconstruction strategy considered defect characteristics such as cause (postoperative), type (partial thickness), location (medial), surface area (1,800 cm2), availability of surrounding soft tissue (moderate), as well as the patient’s systemic comorbidities. For all of the above, we chose the tissue expansion technique.
Byrd and Hobar described a method of expanding skin, fascia, muscle, and peritoneal layers of the abdominal wall using temporarily implanted expanders as a precursor to abdominal wall reconstruction in congenital and post-traumatic defects [8]. Tissue expansion allows the development of well-vascularized, autologous fasciocutaneous flaps that can be advanced to close a midline defect [8]. This technique is rarely used in abdominal wall reconstruction and is reserved for circumstances of significant loss of skin or anterior fascia and when patients are not candidates for component separation, locoregional flaps, or free flaps, such as in our case [2]. Another advantage of this technique is the formation of capsular tissue around implants, which provides an additional layer of highly vascularized tissue and a good background for wound healing [9]. Similarly, we propose an autologous scaffold to reinforce the abdominal wall closure.
The major reported complications of tissue expansion in abdominal wall reconstruction are hematoma (7.3%), expander failure (5.3%), and infection (4.7%) [10]. We did not have any complications with the use of TE in the suprafascial plane.
Free tissue transfer is a reliable alternative to provide adequate coverage for large defects that are not amenable to locoregional flaps [7]. However, it requires the availability of microsurgery and involves donor site morbidity. In this case, the size of the defect prevented its indication (anterolateral thigh flap, latissimus dorsi flap, tensor fascia lata flap, or rectus femoris flap).
Until now, the algorithms proposed to guide the surgeon in the reconstruction of the abdominal wall have not described the optimal technique to repair a partial defect that occupies the entire midline of the abdominal wall, therefore, this case represents a real challenge [1,2].
Conclusions
Tissue expansion remains a viable and safe alternative for patients with complex abdominal wall defects who are not candidates for other reconstruction techniques. This technique allows one to obtain sufficient skin and subcutaneous tissue to provide reliable and well-vascularized coverage for definitive closure. Cosmetic and functional results are satisfactory in terms of skin texture and colour, and long--term stability of the abdominal wall, respectively.
Roles of authors
René M. Palacios Huatuco, and Tatiana Ruffa – performed the literature search, drafted the manuscript, and wrote the final version of the manuscript;
Horacio F. Mayer, and Hernán A. Aguilar – contributed to the surgical treatment of the patient and to the review of the manuscript.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The local Research ethics committee has confirmed that no ethical approval is required for this case report.
Patient consent: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Sources
1. Rohrich RJ., Lowe JB., Hackney FL., et al. An algorithm for abdominal wall reconstruction. Plast Reconstr Surg. 2000, 105 (1): 202–216.
2. Lowe JB 3rd. Updated algorithm for abdominal wall reconstruction. Clin Plast Surg. 2006, 33 (2): 225–240.
3. de Castro CC., Costa Aboudib JH., Salema R., et al. How to deal with abdominoplasty in an abdomen with a sear. Aesthetic Plast Surg. 1993, 17 (1): 67–71.
4. Rieger UM., Erba P., Kalbermatten DF., et al. An individualized approach to abdominoplasty in the presence of bilateral subcostal scars after open gastric bypass. Obes Surg. 2008, 18 (7): 863–869.
5. Zanin EM., Portinho CP., Stensmann IC., et al. Severe and massive necrosis following high definition power-assisted liposuction: a case report. Eur J Plast Surg. 2020, 43 (5): 665–670.
6. Van Geffen HJ., Simmermacher RK. Incisional hernia repair: abdominoplasty, tissue expansion, and methods of augmentation. World J Surg. 2005, 29 (8): 1080–1085.
7. Bauder A., Othman S., Asaad M., et al. Microvascular free tissue transfer for reconstruction of complex abdominal wall defects. Plast Reconstr Surg. 2022, 149 (1): 74e–78e.
8. Hobar P., Rohrich R., Byrd H. Abdominal-wall reconstruction with expanded musculofascial tissue in a posttraumatic defect. Plast Reconstr Surg. 1994, 94 (2): 379–383.
9. Mayer HF, Loustau HD. Capsular grafts and flaps in immediate prosthetic breast reconstruction. Aesthetic Plast Surg. 2014, 38 (1): 129–138.
10. Langdell HC., Taskindoust M., Levites HA., et al. Systematic review of tissue expansion: utilization in non-breast applications. Plast Reconstr Surg Glob Open. 2021, 9 (1) : e3378.
René M. Palacios Huatuco, MD
Plastic Surgery Department
Hospital Italiano de Buenos Aires
4190 Peron St., 1st floor (C1991ABB)
Buenos Aires, Argentina
e-mail: manuel.palacios@hospitalitaliano.org.arSubmitted: 2. 1. 2024
Accepted: 7. 2. 2024Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2023 Issue 3-4-
All articles in this issue
- Editorial
- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Scalp arteriovenous malformations – 20 years of experience in a tertiary healthcare centre
- The comparison of effectivity in breast cancer prevention between skin sparing and subcutaneous mastectomy – 20 years of experience
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- A primary cutaneous carcinosarcoma of the retro auricular region, how to treat and literature review
- Combination of cable ties and barbed sutures for fasciotomy closure – two case reports
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
- Abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with subcostal scars – case report
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

