-
Medical journals
- Career
NANOFIBERS FROM POLYVINYL ALCOHOL AND CAN ENLARGE THE SET OF TRAPPED ODOROLOGICAL TRACES
Authors: Martin Kralovic; Bruno Sopko; Petr Novotny; Evzen Amler
Published in: Lékař a technika - Clinician and Technology No. 4, 2018, 48, 131-135
Category:
Overview
We tested collecting abilities of nanofibers prepared from polyvinyl alcohol and calcofluor (PVAC) for an odorological trace collection. Our study revealed that spectrum of odorological traces trapped on nanofibrous PVAC adsorbents, is more stable and reproducible compared to commonly used ARATEXTM. In addition, both PVA and PVAC nanofibers can adhere a large number of traces undetectable by ARATEX. Our results show that functionalization of commonly used adsorbent ARATEXTM with PVA and PVAC nanofibers can vastly increase the accuracy of trace collection and, thus, significantly improve forensic investigations.
Keywords:
chitin – chitosan – forensic mycology
Introduction
The skin, as the human body’s largest organ, is colonized by numbers of diverse microorganisms such as bacteria, fungi, microscopic mites and others. In another words, a skin microbiome is individually spe-cific [1–3] due to sweat composition, used cosmetic products [4], etc. Skin microbial populations release a lot of various specific metabolites into the surrounding environment contributing, thus, to specificity of the individual human odour [5]. Clearly, this provides an efficient potential source of information for forensic investigation and personal identification for the criminal praxis. Unfortunately, highly sensitive and specific adsorbents are still missing.
This study has been focused on fungal metabolites, especially chitin and chitosan molecules. Clearly, fungal metabolites are an important part of any human odoro-logical trace. Fungi known as abundantly invading human skin, contain chitin and chitosan as a natural part of their cell wall. Fungi have also a high number of chitinases, enzymes metabolizing chitin a chitosan and, thus, producing a diverse spectrum of chitin and chitosan metabolites [6, 7]. The metabolites could be collected and identified.
We have tested nanofibers from a mixture of PVA (polyvinyl alcohol) and calcofluor (PVAC). Calcofluor is a fluorescent dye known as binding chitin and chitosan molecules, and, thus, commonly used as a fluorescent stain for rapid detection of fungi and other microorganisms [8]. We have studied specificity and capacity of PVAC nanofibers to trap odorological traces and compared them with PVA nanofibers and ARATEXTM, used as the golden adsorbent standard in criminology [9, 10].
Material and methods
Collectors and their characterization
ARATEXTM was obtained from CHLUM-TEX LLC. Samples of PVA adsorbent and PVAC were obtained as a present from commercially available source. To determine average nanofiber diameters and adsorbent structure, electron scanning microscopy (SEM) was used. Fibrous samples were coated with a thin layer of gold using a rotatory pumped coater Quorum Q 150R S device (Quorum Technologies, Lewes, UK) and analyzed by SEM (Vega 3, Tescan). To determine average fiber diameters, 100 fibers were randomly chosen and fiber diameter was measured using Tescan software and then the average value was measured and calculated.
Collection of odour traces
A hand pump with defined volume (400 mL) was employed for collection of odour traces. We put 0.35 g sample of the studied adsorbent into the pump chamber.
The pump chamber was connected by a silicone hose to the 750 mL glass jar containing the culture of the black bread mold (Rhizopus stolonifera). During the experi-ment, the glass jar was slightly open to prevent vacuum forming and stop drawing air from the glass jar. Each sample was exposed to 4 000 mL of air from the glass jar with the mold culture. The exposure time was one minute. We prepared 5 samples for each adsorbent, ARATEXTM, PVA and PVAC. Treated adsorbents were stored separately in 50 mL sterile tubes (Corning®) for following chemical analysis.
Analysis
The prepared samples were analyzed by liquid chromatography and mass spectroscopy to determine molecules adsorbed on the adsorbent surface. Prepared samples were incubated in 3 ml of methanol. After centrifugation, 100 µl of the extract was diluted in 900 µl of water and analyzed. The chromatographic separation of analytes was achieved by Agilent 1290 Infinity UHPLC system with a C18 column (Zorbax Eclipse Plus C18 RRHD; 2.1 mm × 100 mm; 1.8 µm; Agilent Technologies). 5mM ammonium formate with 0.01% formic acid (A) and methanol containing 0.01% formic acid (B) were chosen as mobile phases and gradient elution has been used. The Q-TOF mass spectrometer (Agilent 6550 QTOF) with positive and negative electrospray ionisation was used for the untargeted analysis. We used Agilent MassHunter soft-ware to exact masses and the probable summary formulas of detected signals were created.
Statistics
The statistical analyses were carried out using the R program with plots and vegan packages.
Results
Microscopic analysis of PVA and PVAC adsorbent showed and proved submicron fiber diameters.
The average fiber diameter was determined as 0.65 ± 0.32 µm for PVA fibers, and 0.72 ± 0.37 µm for PVA fibers enriched with calcofluor. In contrast to PVA and PVAC adsorbents, ARATEXTM was consisted from microfibers with average diameter 14.1 ± 4.7 µm. Thus, PVA and PVAC adsorbent fibers are approximately 20 times thinner than fibers of ARATEXTM.All samples were exposed to traced molecules as described in Methods, and adsorbed molecules were analyzed. We have identified 1043 different molecules interacting with our sorbents. Figure 1 shows the graphical representation (heatmap) of concentrations of molecules adsorbed on our sorbents, with the cluster analysis. Clearly, the spectrums of adsorbed molecules significant differed. This analysis is even better shown and presented in Figure 2 depicting the results of RDA analysis, where each adsorbent is characterized by different distribution in the reduced dimension scale.
1. The heatmap with detected compounds above 1.2 mil interacting with the sorbent. The scale is in decadic logarithm. For the heatmap which depicts all detected molecules - see The Appendix. 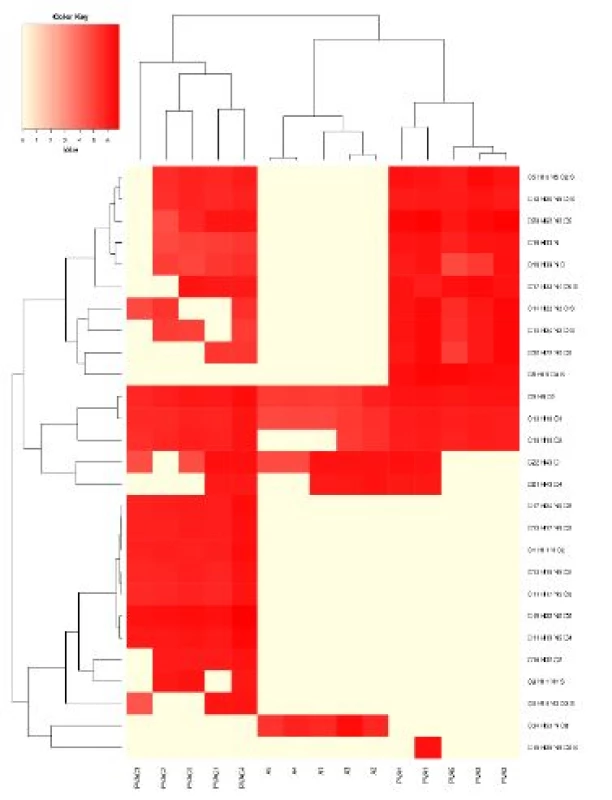
2. The results of the RDA analysis of the concentrations of interacting molecules with our PVA, PVAC nanofiber samples and ARATEX, respectively. 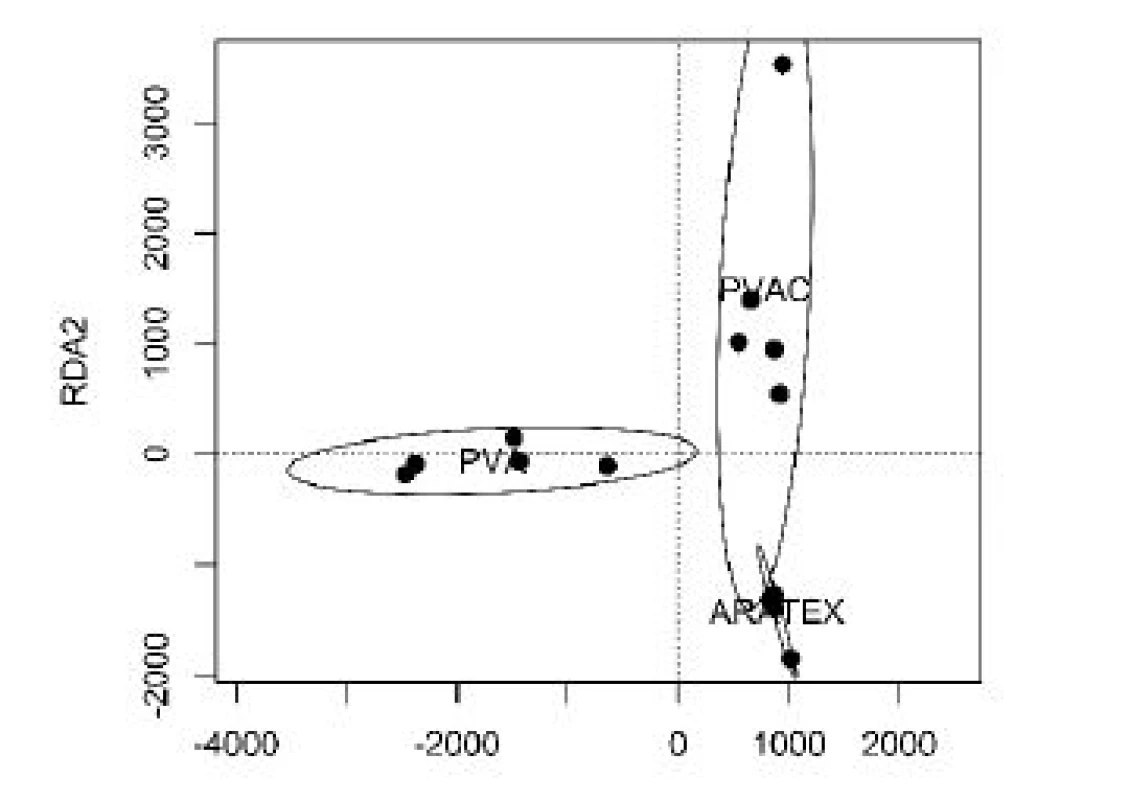
To quantify trapped molecules, the Venn’s diagram of number of molecules detected at least once was prepared (Figure 3). In comparison, 472 unique molecules interacted with ARATEXTM (of those 341 exclusively) 392 molecules (232 exclusively) interacted with PVAC and 403 molecules (219 exclusively) were adsorbed on PVA nanofiber samples.
3. Venn’s diagram of the molecules detected trapped on the sample at least once. PVAC stands for polyvinyl alcohol nanofibers enriched with calcofluor, A (Aratex) and PVA for nanofibers from polyvinyl alcohol. 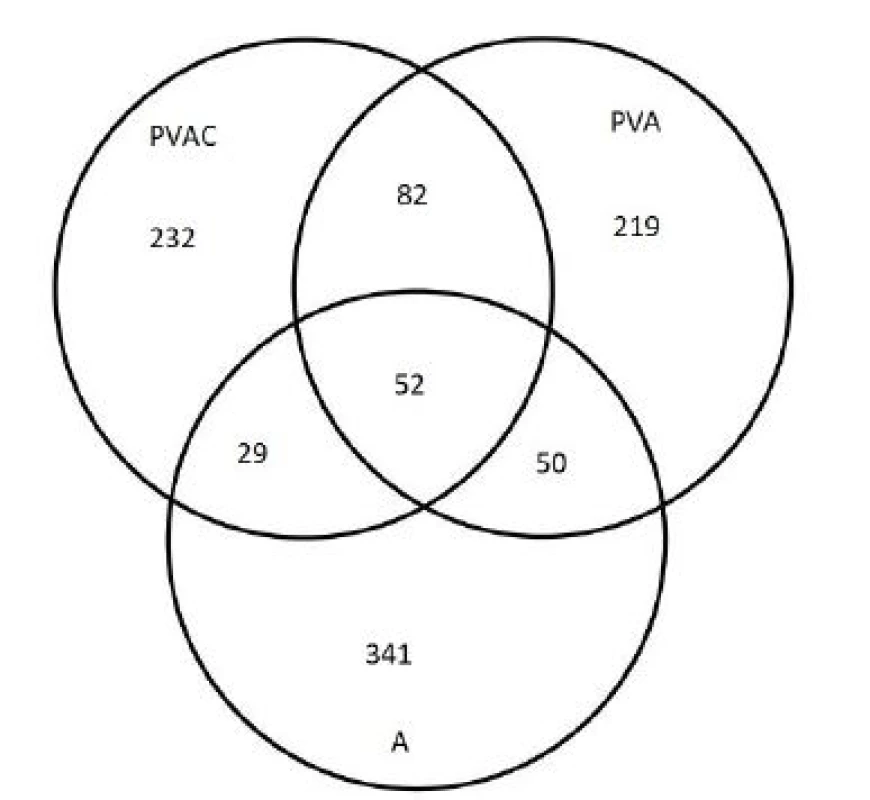
Discussion
The suitability of ARATEXTM, the golden standard for odour trace collection, to collect effectively metab-olites from air has been tested in this study. The obtained results were compared with novel PVA and PVAC nanofiber-based adsorbents. We have proved that ARATEXTM has only limited abilities to trap a full spectrum of molecules. In addition, the total number of trapped molecules by ARATEXTM was comparable to nanofibrous samples despite of a significantly higher mass of compared to nanofibrous PVAC and PVA samples. We suppose that this observation reflects a significantly larger specific surface area of nano-fibrous samples compared to ARATEXTM. This, naturally, opens a possibility for next development of nanofibrous samples from a more complex polymer composition to substitute ARATEXTM. Nanofibrous adsorbent would have plenty of advantages compared to the traditional adsorbent. First, nanofibers are fabricated from well soluble molecules. Thus, a significantly lower adsorbent mass would significantly decrease a back-ground for any liquid chromatography analysis which has been shown as a more sensitive for analysis compared to a gas chromatography. Second, PVA and PVAC nanofiber-based adsorbents showed a different spectrum of entrapped molecules. Thus, we can expect that next development of nanofiber-based adsorbent from a set of several suitable polymers would fully substitute currently employed ARATEXTM in forensic analysis. Third, nanofibrous adsorbents significantly enlarged the number of entrapped molecules. In case of combining all three adsorbents, the number of detected molecules can rise from 472 to 1043 giving the rice of accuracy up to 10343.
Last but not at least, there is the possibility of nano-fibers providing us with the tool for investigation of our skin microbiome.
Conclusion
Our results show that the traditionally used odour trace collection methodology could be significantly improved by application of suitable nanofibrous material. Novel nanofiber-based adsorbents could be prepared and are currently under development in our laboratories. Another option is functionalization of ARATEXTM with PVA and PVAC nanofibers. This could increase the accuracy of trace collection and, thus, significantly improve the forensic investigation. There are several ways how to functionalize ARATEXTM
with PVA and PVAC nanofibers as our preliminary experiments have shown. Functional samples are currently under development.Appendix 1. The heatmap of mostly abundant detected compounds interacting with the sorbent. The scale is in decadic logarithm. 
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
This work has been supported by the Ministry of the Interior within, project No. VI20152018010. We thank Martin Kuchař, Petra Mikšátková, Edita Kofroňová and Gabriela Korbelová for important help with chemical analysis and fibers analysis.
Martin Kralovic, MSc.
Quality of Indoor Environment
University Center for Energy Efficient Buildings
Czech Technical University in Prague
Třínecká 1024, CZ-273 43 Buštěhrad
E-mail: martin.kralovic@cvut.cz
Phone: +420 224 356 735
Sources
- Peterson, J., Garges, S., Giovanni, M., McInnes, P., Wang, L., Schloss, J. A., Bonazzi, V., McEwen, J. E., Wetterstrand, K. A., Deal, C., Baker, C. C.: The NIH human microbiome project. Genome Res., 2009; vol. 19, no. 12, pp. 2317–23.
- Grice, E. A., Segre, J. A.: The skin microbiome. Nat. Rev. Microbiol. 2011; vol. 9, no. 4, pp. 244.
- Findley, K., Oh, J., Yang, J., Conlan, S., Deming, C., Meyer, J. A., Schoenfeld, D., Nomicos, E., Park, M., Kong, H. H, Segre, J. A. Human skin fungal diversity. Nature. 2013; vol. 498, no. 7454, pp. 367.
- Grice, E. A., Kong, H. H., Conlan, S., Deming, C. B., Davis, J., Young, A. C., Bouffard, G. G., Blakesley, R. W., Murray, P. R., Green, E. D., Turner, M. L.: Topographical and temporal diver-sity of the human skin microbiome. Science. 2009; vol. 324, no. 5931, pp. 1190–1192.
- Rennie, P. J., Gower, D. B., Holland, K. T., Mallet A. I., Watkins, W. J.: The skin microflora and the formation of human axillary odour. Int J Cosmet Sci. 1990; vol. 12, no. 5, pp. 197–207.
- Seidl, V.: Chitinases of filamentous fungi: a large group of diverse proteins with multiple physiological functions. Fungal Biol Rev. 2008; vol. 22, no. 1, pp. 36–42.
- Hartl, L., Zach, S., Seidl-Seiboth, V.: Fungal chitinases: diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. and Biotechnol.. 2012; vol. 93, no. 2, pp. 533–543.
- Monheit, J. E., Cowan, D. F., Moore, D. G.: Rapid detection of fungi in tissues using calcofluor white and fluorescence micro-scopy. Arch. Pathol. Lab. Med. 1984; vol. 108, no. 8, pp. 616–618.
- Vyplelová, P., Vokálek, V., Pinc, L., Pacáková, Z., Bartoš, L., Santariová, M., Čapková, Z.: Individual human odor fallout as detected by trained canines. Forensic. Sci Int. 2014; vol. 234, pp. 13–15.
- Pinc, L., Bartoš, L., Reslova, A., Kotrba, R.: Dogs discriminate identical twins. PLOS One. 2011; vol. 6, no. 6, e20704.
Labels
Biomedicine
Article was published inThe Clinician and Technology Journal

2018 Issue 4-
All articles in this issue
- Computer-aided modeling and additive manufacturing fabrication of patient-specific mandibular implant
- Immediate effect of physical on blood flow velocity in radial artery in young adults
- Nanofiber scent carrier
- NANOFIBERS FROM POLYVINYL ALCOHOL AND CAN ENLARGE THE SET OF TRAPPED ODOROLOGICAL TRACES
- GLOBAL CENTERS OF MEDICAL DEVICE TECHNOLOGY: UNITED STATES, EUROPE AND CHINA
- The Clinician and Technology Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- GLOBAL CENTERS OF MEDICAL DEVICE TECHNOLOGY: UNITED STATES, EUROPE AND CHINA
- Computer-aided modeling and additive manufacturing fabrication of patient-specific mandibular implant
- Nanofiber scent carrier
- Immediate effect of physical on blood flow velocity in radial artery in young adults
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

