-
Medical journals
- Career
CELL-BASED SENSOR CHIP FOR NEUROTOXICITY MEASUREMENTS IN DRINKING WATER
Authors: Dennis Flachs; Manuel Ciba
Authors‘ workplace: BioMEMS Lab, Faculty of Engineering, University of Applied Sciences Aschaffenburg, Germany
Published in: Lékař a technika - Clinician and Technology No. 2, 2016, 46, 46-50
Category: Original research
Overview
Our drinking water contains residues of pharmaceuticals. A sub-group of these contaminants are neuro-active substances, the antiepileptic carbamazepine being one of the most relevant. For assessment of the neurotoxicity of this drug at a sub-therapeutic level, a cell-based sensor chip platform has been realized and characterized. For this purpose, a microelectrode array chip was designed and processed in a clean room and optimized in terms of low processing costs and good recording properties. For characterization of the system neuronal cells were plated on microelectrode array chips and electrical activity was measured as a function of applied carbamazepine concentration. We found that the relative spike rate decreased with increasing drug concentration resulted in IC50 values of around 36 μM. This value is five orders of magnitude higher than the maximal dose found in drinking water. IC50 values for burst rate, burst duration and synchrony were slightly higher, suggesting spike rate being a more sensitive parameter to carbamazepine.
Keywords:
Microelectrode array, carbamazepine, neurotoxicity, cell-based biosensorIntroduction
Every year large amounts of human and veterinary pharmaceuticals find their way in our drinking water cycle. With new and improved analytic methods these drugs can be detected – even in very low concentrateons. These new options increase the awareness of possible effects correlated to the chronic uptake of drugs within drinking water. Although, it is likely that residues in the water have no adverse effects on the human health, there is no regulation in terms of critical concentration values [1] due to a lack of data regarding chronic exposure at low doses.
The number of pharmaceutically active substances found in drinking water is surprisingly high. More than twenty different active components and their metabolites have been detected in drinking water [1]. Tab. 1 shows some examples of detected drug in drinking water. In this work we focused on neuro-effective drugs as they have hardly been addressed in the literature yet. Therefore, we searched literature for measurements of neuro-effective drugs found in drinking water [1, 2]. Typical compounds are diazepam, diclofenac, and carbamazepine.
1. Some examples of drugs detected in drinking water and their maximal concentration cmax. 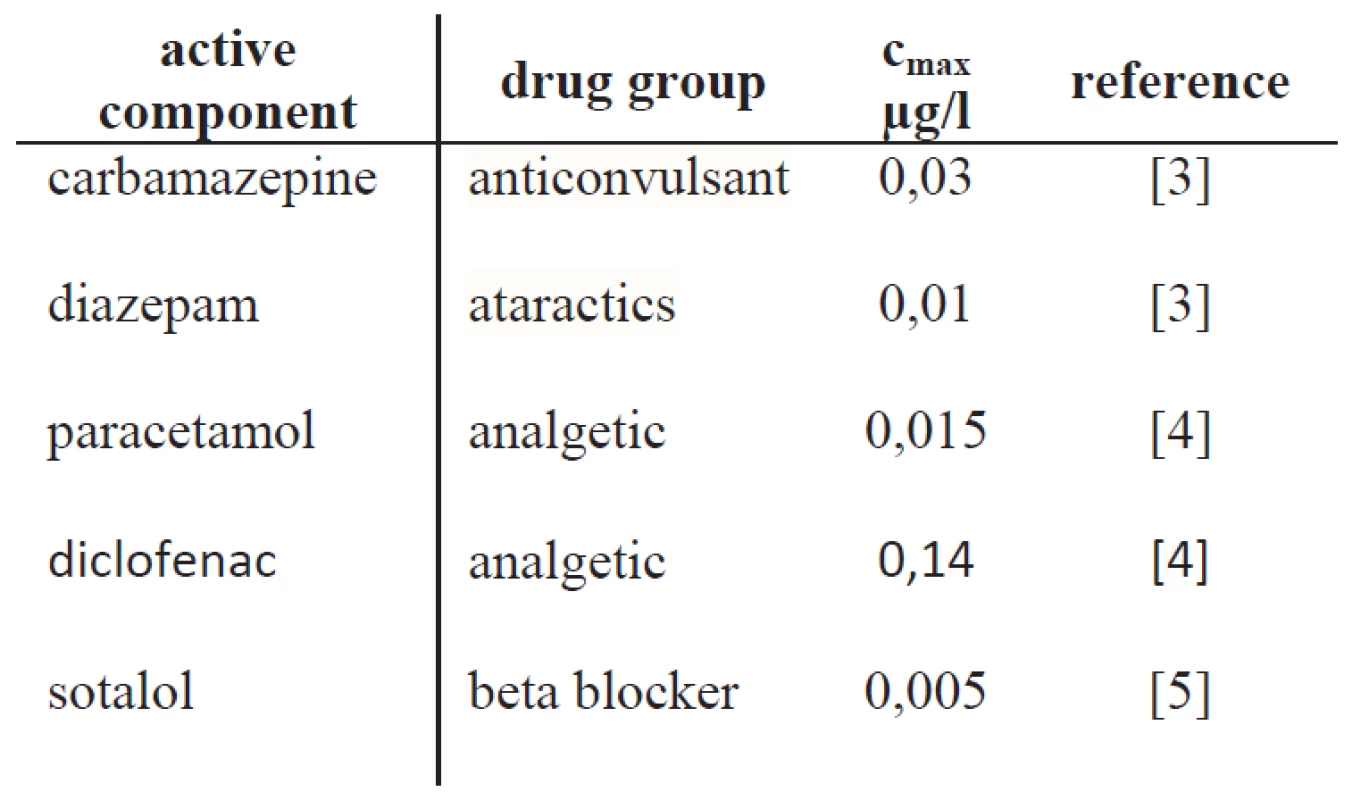
The most relevant substance identified was carbamazepine. Carbamazepine (CBZ) is a medication used primarily in the treatment of epilepsy and neuropathic pain and it is well known to reduce electrophysiological activity of the brain by blocking voltage-gated sodium channels [6]. Also, the activation of GABAA receptors were reported [7]. In Germany, CBZ has been identified at twenty-six different samples with a maximum concentration of 0.03 μg/l (0.13 nM) [1]. Obviously, the measured concentrations depend strongly on country and the area where samples were taken. Maximum CBZ concentration in efflux of German sewage treatment plants were 6.3 µg/l [8], whereas higher concentrations of up to 27.27 µg/l have been found in the efflux of sewage treatment plants in Cyprus, which leads to the assumption that drinking water concentration in that region could be even higher [9]. Our hypothesis is that CBZ, if incorporated on a regular base – even at sub-therapeutic levels – has the potential to induce unwanted changes in the brain’s functionality.
With this background, we propose the application of a cell-based in vitro assay to assess the neurotoxicity of CBZ and as a perspective other neurotoxins relevant for drinking water. For this purpose a cell-based sensing platform shall be developed. Microelectrode array (MEA) chips coupled to neuronal cell networks are a common approach to achieve a basic under-standing of electrophysiological properties of neuronal networks [10, 11]. Electrical activity of a neuronal network is recorded in vitro and changes related to the application of a neuroactive substance are analyzed. With this goal, we fabricated MEA chips with gold electrodes to record electrophysiological signals of a cortical neuronal network and characterized the cell-based sensing platform for the drug CBZ.
Materials and Methods
MEA chip processing
The microelectrodes for recordings of neuronal signals have been processed of various metals or conduction polymers [12]. In this study a MEA chip was processed with gold microelectrodes. Fig. 1 shows the MEA chip and its microelectrode layout. The outer size of the glass chip is 45×45 mm providing 59 microelectrodes with a diameter of 30 μm (and a spacing of 200 μm) and a reference electrode. All electrodes are connected via conducting lanes to large metal pads at the outer boarder of the chip, while insulation and biocompatibility are provided by a thick SU8 resist. To improve the impedance, the electrodes may be coated with nanowires or carbon black to increase the surface area of the electrodes. The glass ring on top of the MEA chip builds a culture chamber of about 1.5 ml.
Fig. 1: MEA chip with glass ring (left) and its magnified microelectrode array (right). The microelectrode array consists of 59 microelectrodes. A larger reference electrode is located inside the glass ring area next to the microelectrode array. 
Neuronal cell culture
Neuronal cell culturing was performed by using cryo-conserved embryonic cortical rat neurons (E18 and E19) (Lonza Ltd, Basel, Switzerland). Cell culture medium was prepared according to the following protocol: L-glutamine (2 mM) and 2% NSF-1 were added to the PNBM basal medium. NSF-1, a supplement supporting neuronal growth and survival, was aliquoted, frozen and added to the medium immediately before each use. Neurons were thawed and cultured with a density of approximately 5.000 cells/mm2 onto each substrate and incubated in a humidified atmosphere for 4 h. Finally, petri dishes were filled with approximately 1.5 ml culture medium. The Medium was fully replaced twice a week.
Electrophysiological recording
Neuronal signals were recorded extracellularly at a sampling rate of 10 kHz with a MEA60 amplifier system (Multichannel System, Reutlingen, Germany) and stored for offline analysis. Recordings were performed outside the incubator. To maintain the sample temperature at 37°C, a temperature-controller (TC01, Multichannel Systems, Reutlingen, Germany) was employed during the recordings. Controlled CO2 atmosphere (5%) was provided by a custom-made mini incubator and a CO2 controller (CO2-Controller 2000, Pecon, Erbach, Germany).
Data analysis
Raw data were analyzed using custom-made Matlab (The Math Works Inc., Natick, Massachusetts, U.S.A.) based software tool Dr. Cell featuring spike detection using a threshold-based algorithm, with a threshold calculated by multiplying the standard deviation of the noise by a factor of -5 [12]. Fig. 2 shows a typical raw signal and its corresponding spike train which is used for analysis. Shortly successive occurring spikes were defined as burst, if the burst consists of at least three spikes with times between each spike not exceeding 100 ms (“mini-bursts”) [13]. Synchrony of the neuronal network was measured by binary binning each spike train into 500 ms bins, calculating cross-covariance without delay between all pairs of spike trains, and averaging those values [14].
Fig. 2: Nine electrodes are displayed (electrode diameter 30 μm) including neuronal network. Right: Raw neuronal signal recorded from one electrode featuring spikes whose occurring times (spike train) are used for analysis. 
Statistics
Data (given as mean ± standard deviation of N samples) were collected from different cell cultures. Data points of dose-response curves from electrophysiological recordings were fitted using the Hill equation
where c donates the concentration of the test substance, c0 the half maximal effective concentration (designated as EC50 for excitation and IC50 for inhibition), NH the Hill coefficient, and A1 and A2 the spike rate under control conditions and saturated concentration of the test substance.
Significant changes from control condition were assessed using parameter free Kruskal-Wallis test. Multiple testing was performed using Bonferroni correction.
Drugs
Test substance CBZ (CAS number 298-46-4; C4024-1G, Sigma Aldrich, St. Louis, USA) was diluted in dimethyl sulfoxide (DMSO) (CAS number 67-68-5; Sotrachem, Saint Denis, France) and was manually applied to the medium. CBZ concentrations of 2 μM, 20 μM, 40 μM, 60 μM, 100 μM, 200 μM and 1000 μM were added accumulatively and recorded for 5 minutes each. Maximum DMSO concentration at 1000 μM CBZ was 0.5%, which has been shown to have no significant effect on neural activity [6].
Results and Discussion
Typical patterns of spike trains in the absence and presence of CBZ are displayed in Fig. 3. Spike rate in the absence of CBZ were around 500 spikes per minute per electrode. Fig. 4 shows CBZ concentration dependent parameter like spike rate (Fig. 4a), spike amplitudes (Fig. 4b), burst rate (Fig. 4c), burst duration (Fig. 4d) and spike synchrony (Fig. 4e) normalized to the control condition (absence of CBZ). Data are displayed as mean ± standard deviation. Except for spike synchrony, normalized parameter were calculated for every active electrode and subsequently averaged across the chip. Fitting of the spike rate with Hill equation resulted in an IC50 value of 38 μM which is in agreement with values reported for primary murine frontal cortex neurons [10]. IC50 values for burst rate, burst duration and synchrony were slightly higher suggesting spike rate as the most sensitive parameter to capture the effect of CBZ. Concentrations of 60 µM and higher caused statistical significant effects compared to control condition for spike rate (p<0.05), burst rate (p<0.05), burst duration (p<0.01) and synchrony (p<0.01). Spike amplitude decreased not before 100 µM and was therefore the most insensitive parameter to CBZ.
Fig. 3: Spike trains of six electrodes recorded from a MEA chip are shown in the absence of CBZ (0 μM) and the presence of CBZ (40 μM and 100 μM). With increasing CBZ concentration neuronal activity decreases. 
Fig. 4: Effects of increasing concentrations of CBZ on the spike rate (a), spike amplitude (b), burst rate (c), burst duration (d), and spike synchrony (e) of neurons coupled with MEA chips (N= 6, 21–25 div). Data points represent the mean ± standard deviation and were normalized to the control at zero concentration (not shown in the diagram). Data are fitted using the Hill equation to calculate half maximal inhibitory concentration (IC50). Significant changes from control condition assessed with Kruskal-Wallis test followed by Bonferroni corrected multiple testing, are indicated by *, P<0.05 and **, P<0.01. 
Compared to concentrations measured in drinking water, IC50 values and statistical significant acute effects are five orders of magnitude higher. Therefore, no acute effects on neurons are expectable due to exposure of CBZ in concentrations measured in drinking water. Since CBZ exposure over two weeks showed GABA-receptor upregulation in vivo [15] this effect could be also expected at neurons on MEA chips. Since neurons coupled to MEA chips can be cultured for several weeks, long-term exposure is possible and can lead to significant alterations in spontaneous activity like shown for mercury [16]. Even if no alteration in spontaneous activity is observable, acute experiments after long-term exposure may reveal long-term effects. For example an application of the allosteric GABAA inhibitor bicuculline could change the signal activity differently of control and exposed neurons [17]. Using this strategy, identifying a concentration not causing any long-term effects could be used to define a threshold value for drinking water. Moreover, neurons coupled to MEA chips might be also applied to study acute and long-term effects of certain mixtures of pharmaceuticals found in drinking water.
Conclusion
We realized an in vitro cell-based sensing platform in order to assess neurotoxicity of active components that are relevant contaminants of drinking water.
Neurons coupled to MEA chips were electrical active and signals could be recorded and analyzed. The acute application of the neuroactive substance CBZ caused a concentration dependent decrease in spike rate with an IC50 value of around 36 μM, which was expected [11]. Further experiments will now address the chronic application of CBZ for several days and weeks.
Acknowledgement
Research described in the paper was supervised by Prof. C. Thielemann, biomems lab, UAC Aschaffenburg and financially supported by the Bayrisches Staatsministerium für Bildung, Kultus, Wissenschaft und Kunst in the frame of FH Forschungsschwerpunkt. We would like to thank Dr. CRISTOPH NICK for the making of the MEA chip layout.
Dennis Flachs, B. Eng.
BioMEMS Laboratory
Faculty of Engineering
University of Applied Sciences in Aschaffenburg
Würzburger Straße 45, 63743 Aschaffenburg, Germany
E-mail: dennisflachs@web.de
Manuel Ciba, M. Eng.
BioMEMS Laboratory
Faculty of Engineering
University of Applied Sciences in Aschaffenburg
Würzburger Straße 45, 63743 Aschaffenburg, Germany
Sources
[1] Bergmann, A., Fohrmann, R., Weber, F.-A. Zusammenstellung von Monitoringdaten zu Umweltkonzentrationen von Arznei-mitteln. Umweltbundesamt, 2011.
[2] Webb, S., Ternes, T., Gibert, M., Olejniczak, K. Indirect human exposure to pharmaceuticals via drinking water. Toxicology letters, 2003, vol. 14, no.3, p. 157–167.
[3] Bergmann, A., Fohrmann, R., Hembrock-Heger, A. Bewertung der Umweltrelevanz von Arzneistoffen. Umweltwissenschaften und Schadstoff-Forschung, 2008, 20(3):197–208.
[4] Boutrup, S., Jaakko, M., Dam, M., Mønster, T. PPCP monitoring in the Nordic Countries - Status Report. Nordic Council of Ministers, 2012.
[5] Rönnefahrt, R., Amato, Ebert, I., Schönfeld, J. Arzneimittel in der Umwelt–Ein Risiko? UMID: Umwelt und Mensch–Informationsdienst, 2012, 1 : 36–43.
[6] Sheets, P. L., Heers, C., Stoer, T., Cummins, T. R. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. Journal of Pharmacology and Experimental Therapeutics, 2008, vol. 326, no. 1, p. 89–99.
[7] Liu, L., et al. The mechanism of carbamazepine aggravation of absence seizures. Journal of Pharmacology and Experimental Therapeutics, 2006, 319.2, 790-798.
[8] Ternes, T. A. Occurrence of drugs in German sewage treatment plants and rivers. Water research, 1998, 32.11, 3245-3260.
[9] Fatta-Kassinos, D., et al. Existence of pharmaceutical compounds in tertiary treated urban wastewater that is utilized for reuse applications. Water resources management, 2011, 25.4, 1183-1193.
[10] Johnstone, A. F., et al. Microelectrode arrays: a physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology, 2010, vol. 31, no. 4, p. 331–350.
[11] Daus, A.W., Layer P.G., Thielemann, C. A spheroid-based biosensor for the label-free detection of drug-induced field potential alterations. Sensors and Actuators B: Chemical, 2012, vol. 165, p. 53-58.
[12] Nick, C., Goldhammer, M., Bestel, R., Steger, F., Daus, A. W., Thielemann , C. DrCell – A Software Tool for the Analysis of Cell Signals recorded with Extracellular Microelectrodes. Signal Proc Int J, 2013, vol. 7, no. 2, p. 96-109.
[13] Baker R. E., Corner M. A., Pelt J. Spontaneous neuronal discharge patterns in developing organotypic mega-co-cultures of neonatal rat cerebral cortex. Brain research, 2006, 1101(1):29–35.
[14] Selinger, J. V., Pancrazio, J. J., Gross, G. Measuring synchronization in neuronal networks for biosensor applications. Biosensors and Bioelectronics, 2004, 19.7, 675-683.
[15] Motohashi, N., Ikawa, K., Kariya, T. GABAB receptors are up-regulated by chronic treatment with lithium or carbamazepine. GABA hypothesis of affective disorders. European journal of pharmacology, 1989, 166(1):95–99.
[16] Gopal, K. V. Neurotoxic effects of mercury on auditory cortex networks growing on microelectrode arrays: a preliminary analysis. Neurotoxicology and teratology, 2003, 25(1):69–76.
[17] Hogberg, H. T., et al. Application of micro-electrode arrays (MEAs) as an emerging technology for developmental neurotoxicity: evaluation of domoic acid-induced effects in primary cultures of rat cortical neurons. Neurotoxicology, 2011, 32(1):158–168.
Labels
Biomedicine
Article was published inThe Clinician and Technology Journal

2016 Issue 2-
All articles in this issue
- CROSSOVER FROM AUTOMATED TO MANUAL TITRATION OF FiO2 IN THE NICU: IS THERE A TRANSITION LAG?
- DESIGN CONCEPTS FOR PREVENTING GAS BUBBLE INTERFERENCE IN MICROFLUIDIC DEVICES
- CELL-BASED SENSOR CHIP FOR NEUROTOXICITY MEASUREMENTS IN DRINKING WATER
- TESTING A SYSTEM FOR PREDICTING MICROSLEEP
- EARLY DISCHARGE (48–72 HOURS) AFTER ACUTE ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION: INTERIM RESULTS OF THE OPEN, RANDOMIZED, MONOCENTRIC STUDY
- The Clinician and Technology Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- EARLY DISCHARGE (48–72 HOURS) AFTER ACUTE ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION: INTERIM RESULTS OF THE OPEN, RANDOMIZED, MONOCENTRIC STUDY
- CELL-BASED SENSOR CHIP FOR NEUROTOXICITY MEASUREMENTS IN DRINKING WATER
- TESTING A SYSTEM FOR PREDICTING MICROSLEEP
- DESIGN CONCEPTS FOR PREVENTING GAS BUBBLE INTERFERENCE IN MICROFLUIDIC DEVICES
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


