-
Medical journals
- Career
Blank spaces in the management of venous thromboembolism prophylaxis at surgical departments and the impact of a clinical pharmacist
Authors: I. Murinova 1,2; M. Kovacic 1; L. Polaskova 1; K. Reveszova 1; L. Schrabalova 1; B. Cermakova; Juráňová 1,3; J. Saloun 2
Authors‘ workplace: Oddělení klinické farmacie, Ústřední vojenská nemocnice – Vojenská fakultní nemocnice, Praha 1; Ústav aplikované farmacie, Farmaceutická fakulta, Masarykova univerzita, Brno 2; Farmakologický ústav 1. lékařská fakulta University Karlovy a Všeobecná fakultní nemocnice Praha 3
Published in: Rozhl. Chir., 2021, roč. 100, č. 12, s. 592-602.
Category: Original articles
doi: https://doi.org/10.33699/PIS.2021.100.12.592–602Overview
Introduction: A venous thromboembolism (VTE) prophylaxis policy should be in place at all hospitals. While professional societies provide general guidance, they do not take into account the full range of procedures in this area. The aim of this study was to fill these “blank spaces” and design a VTE prophylaxis system which would reflect the wide range of surgical procedures.
Methods: We conducted an extensive literature review. Surgeons were subsequently asked to help score procedures according to VTE and bleeding risk while taking into consideration procedure type and duration, surgical techniques, etc.
Results: Clinical pharmacists proposed a standardized VTE prophylaxis protocol. For some surgical fields the Caprini risk assessment model (RAM) was adapted. A unique VTE prophylaxis system was created for surgical fields where Caprini RAM has not been validated. We proposed dosage adjustments for obese patients and for patients with renal impairment. A dedicated software application was developed.
Conclusion: The implementation of the VTE prophylaxis system resulted in a uniform and more rational prescription of pharmacological and mechanical prophylaxis as well as in financial savings. The development of the app increased compliance with the system and, in combination with real-time auditing, significantly improved safe drug administration practices at Military University Hospital Prague. The system is unique in continental Europe and contains wide range of surgical procedures and matching VTE prophylaxis, which has never before been published in this scope. It demonstrates the contribution of clinical pharmacists to the improvement of hospital care quality.
Keywords:
application software − clinical pharmacy − surgical procedure − venous thromboembolism − VTE prophylaxis
INTRODUCTION
Venous thromboembolism (VTE) is a common and preventable complication of surgical and medical hospitalizations. A VTE prophylaxis policy (VTE risk assessment and prophylactic strategy), ideally supported by an application with regular updates and feedback validation, should thus be in place at all institutions [1]. While professional societies provide general guidance, they do not take into account the full range of procedures in this area. We therefore focus on these “blank spaces”, i.e. surgical procedures which are not mentioned in literature or in professional guidelines, but which are regularly performed.
Prior to the establishment of the Clinical Pharmacy Department, VTE prophylaxis at Military University Hospital Prague (MUHP) was inconsistent and prone to many medication errors, including e.g. the widespread and unnecessary administration of low molecular weight heparins (LMWH) in all surgical specialties, the widespread use of mechanical prophylaxis, variable pharmacological prophylaxis duration, inconsistent or no LMWH dose adjustments for obese patients and patients with renal impairment.
Our goal is to design a VTE prophylaxis system which will reflect the wide range of surgical procedures at MUHP, increase compliance with existing guidelines and lead to a more rational prescription of VTE prophylaxis while also resulting in financial savings. We also aim to propose LMWH dosage adjustments for obese patients and patients with renal impairment.
METHODS
We conducted an extensive literature review of papers published between 2001 and 2019 using various combinations of MeSH (Medical Subject Headings) terms in PubMed to acquire an understanding of approaches to VTE prophylaxis in surgical patients and to obtain an overview of national and international professional societies’ recommendations. We also studied strategies to increase compliance with existing guidelines [1−5]. The electronic database UpToDate was used as a secondary source.
General, abdominal, vascular, bariatric, and thyroid surgeons as well as gynaecologists, urologists, orthopaedists, neurosurgeons, and eye surgeons were subsequently asked to help score procedures according to VTE and bleeding risk while taking into consideration procedure type and duration, surgical techniques, etc.
RESULTS
Based on studied literature, we proposed a standardized VTE prophylaxis system. This system included indication of an anticoagulant (LMWH or direct oral anticoagulans, DOAC), administration route, dosage, timing (with respect to surgery, spinal, or epidural anaesthesia), duration and dose adjustments for special populations (renal impairment, obesity). Recommendations were proposed in accordance with the Summary of Product Characteristics.
Approaches to VTE prophylaxis in surgical fields were subdivided into two categories. The first category comprised general, abdominal, pelvic, vascular, and thyroid surgery, gynaecology, and urology, where we adapted the Caprini risk assessment model (Caprini RAM), with some adjustments, for surgery scoring [6−9]. Surgery risk scoring using Caprini RAM is based on operation time with a cut-off value of 45 min. We modified this default criterion, because operation duration does not depend only on the on the extent of the surgery, which determines VTE risk, but also on the experience and skill of the surgeon. In cooperation with surgeons, we classified surgeries into VTE risk groups according to the extent of the surgery, utilized surgical techniques and the risk of complications. Additional risk factors were adapted with only minor changes. The second category included orthopaedics, neurosurgery, and eye surgery, where no specific VTE risk scoring system is recommended; a unique system of VTE prophylaxis was therefore developed. In orthopaedics, the indication of VTE pharmacological prophylaxis depends primarily on the nature of the individual procedure. In neurosurgery, finding a balance between a high risk of VTE and a high risk of bleeding is essential. Based on the site of surgery, bleeding can be fatal to a patient.
The recommended duration of VTE pharmacological prophylaxis was generally established as 7 to 10 days or until a patient becomes fully ambulatory – with the exception of large-scale orthopaedic surgeries, extensive surgeries for malignancy and spinal lesions, where pharmacological prophylaxis is extended. We proposed dosage adjustments for obese patients and patients with renal impairment. In obese patients with total body weight over 110 kg we recommend an increase in LMWH dosage by 0.2 ml and in patients over 130 kg by 0.4 ml. In patients with renal impairment (glomerular filtration below 30 ml/min) we recommend an individual approach and consultation with a clinical pharmacist.
Compliance with the proposed and tested VTE prophylaxis system was increased by the development of dedicated application software (Fig. 1, 2) and real time auditing. Using the web-based app, physicians select a patient’s parameters (age, weight, renal status, additional anamnestic risk factors) and procedure type. The app then evaluates VTE prophylaxis methods and duration for each patient. As the app has been developed for use at the MUHP, it is currently only available in a Czech language version.
Fig. 1: Example of the VTE scoring system application software in use at Military University Hospital Prague 
Fig. 2: Example of the VTE prophylactic strategy application software in use at Military University Hospital Prague 
Multidisciplinary cooperation between clinical pharmacists, physicians and IT specialists led to the development of a standardized VTE scoring system and prophylactic strategy for each surgical field as well as to the development of an app accessible through the hospital information system. The new VTE prophylaxis system is capable of tackling major drug-related problems encountered in everyday clinical practice and facilitate the rapid classification of patients into well-defined groups, as recommended by the American College of Chest Physicians (ACCP) [1].
VTE prophylaxis methods
VTE prophylaxis methods include:
1) General measures (early and frequent ambulation and optimal hydration). Recommended to all patients.
2) Pharmacological prophylaxis with LMWH or DOAC (selected orthopaedic surgery). Fondaparinux is prescribed when heparins are contraindicated (e.g. heparin induced thrombocytopenia). We do not recommend it as a first line agent due to the unavailability of lower strength (used in patients with creatinine clearance 20–50 ml/min) in the Czech Republic and high product price. Unfractionated heparin (UFH) is also available if needed.
3) Mechanical prophylaxis with graduated compression stockings (GCS) or bandages. Intermittent pneumatic compression (IPC), preferred by many authorities [1,2], is not available at our hospital. In mechanical prophylaxis, thigh-length GCS are preferred over knee-length. Correct pressure at ankle level is 18–23 mm Hg [1]. Two types of GCS available in the Czech Republic (18–21 mm Hg and 23–32 mm Hg) meet these requirements. The latter GCS type is preferred at our hospital.
Recommendations by surgical specialty
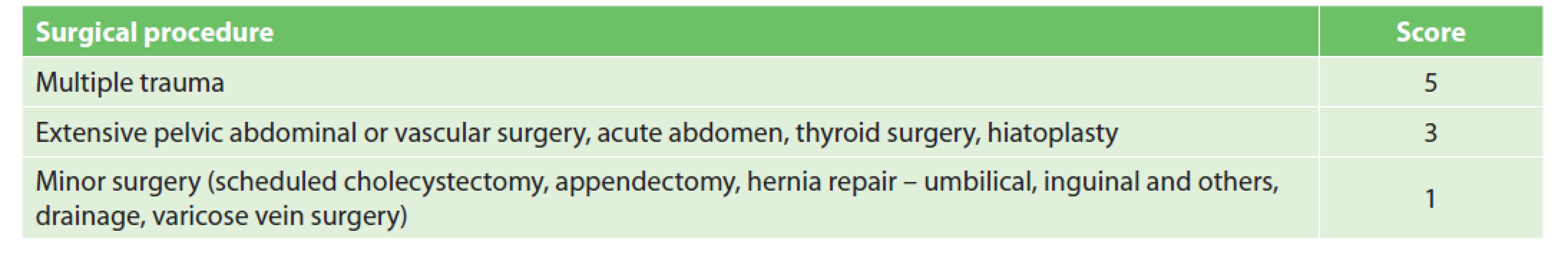
VTE prophylaxis in general, abdominal, vascular, bariatric, and thyroid surgery
We used Caprini RAM for patient stratification. Some risk factors (RFs) were adopted in full while others were modified to suit the conditions at MUHP. VTE prophylaxis recommendations are made based on the assessment of both personal and procedure-related risk factors.
According to Caprini RAM, procedures are assigned either one point (minor surgery <45 min) or two points (major or laparoscopic surgery >45 min). One additional point is recommended for surgeries lasting longer than two hours (including anaesthesia time) [9]. We found that scoring according to operative time is insufficient, as only a small number of surgeries take less than 45 min; furthermore, duration also depends on the experience and skill of the surgeon, not only on the extent of the surgery. Following our agreement with the surgeon, procedures were divided into three groups (Tab. 1). The first group includes low VTE risk procedures (1 point) where pharmacological prophylaxis is generally not required. However, for patients undergoing low-risk procedures with additional RFs (according to Caprini RAM) pharmacological prophylaxis may become indicated [6]. The second group contains high risk procedures (3 points) where pharmacological prophylaxis is indicated regardless of the other RFs. The third group contains polytrauma (5 points) where pharmacological and also mechanical prophylaxis is indicated.
By adding up points for surgery and other RFs, each patient is allocated to one of three groups (low, moderate, and high risk) [1,6]. Pharmacological prophylaxis is not recommended in the low VTE risk group (0–2 points), as it can result in similar numbers of non-fatal VTE events prevented and non-fatal major bleeding events caused (grade 1B) [1]. The use of mechanical prophylaxis in this group of patients is a matter of some debate. Mechanical prophylaxis is recommended by the ACCP and the American Society of Haematology, but with a low level of evidence (grade 2C). While both authorities prefer the use of IPC over GCS [1,10], IPC is unfortunately not available at our institution. According to the ACCP, mechanical prophylaxis may be expected to prevent 8–10 nonfatal VTE events per 1,000 patients at the expense of an uncertain number of skin complications. The absolute number may be even lower in the very low risk group. According to the European Society of Anaesthesiology (ESA), mechanical prophylaxis is not recommended in lowrisk patients [2]. Based on the controversial benefits of GCS in this group of patients, the risk of skin complications, patient inconvenience and higher costs, we agree that only general measures of VTE prophylaxis should be used in this group of patients. Pharmacological prophylaxis of VTE (Grade 1B) without mechanical prophylaxis is recommended to patients who are at moderate VTE risk (3–4 points) [1]. Mechanical prophylaxis is preferred in patients who are at high risk of major bleeding complications. A combination of pharmacological and mechanical prophylaxis (grade 1B, 2C) is recommended for patients at high VTE risk (5+ points) [1]. The addition of mechanical prophylaxis can further reduce the absolute number of non-fatal VTE.
Limited data are available with respect to VTE prophylaxis duration. The risk of VTE remains elevated for at least 12 weeks following surgery [3]. It is generally recommended to continue pharmacological prophylaxis for 7–10 days or until a patient becomes fully ambulatory [2,5,11]. An extension of pharmacological prophylaxis for up to 4 weeks is recommended in abdominal or pelvic surgery for cancer (Grade 1B) [1,2,5]. The most complicated group is one-day surgery (1-point procedures) in patients with additional RFs which classify the patient as a moderate VTE risk; pharmacological prophylaxis is recommended in such cases. No strong evidence-based trials are available for this group. The ESA recommends a minimum of seven days over shorter regimens (Grade 1B), but in certain cases pharmacological prophylaxis could be limited to hospitalization only (Grade 2C) [2]. We believe that the administration of 1–2 doses of LMWH in the case of one-day surgery patients has very low benefit. It is also necessary to take into account that the range and complexity of one-day surgery procedures is expanding and that patients sent home following a brief hospitalization may unexpectedly become immobile due to postoperative pain or weakness and may develop leg swelling due to inactivity [12]. This may increase their chance of suffering a thrombotic event in an outpatient setting. Therefore, even for one-day surgery, we recommend 7–10 days of pharmacological prophylaxis if so indicated.
Prior to the introduction of the new VTE prophylaxis system, nearly all Surgical Department patients received both mechanical and chemical prophylaxis. In the first year following the implementation of the new system, patients were stratified into low risk (20%), moderate (50%) and high risk (30%) groups. The new stratification system significantly reduced LMWH consumption and eliminated mechanical prophylaxis in the low and medium risk group and resulted in significant financial benefits. Direct financial savings associated with GCS reached approximately USD 10,000 per month.
VTE prophylaxis in gynaecology
In gynaecology, as in general surgery, we proceeded according to ACCP recommendations. Most surgical procedures at the Gynaecological Department of MUHP are performed laparoscopically (LPS). Data from literature suggests that laparoscopic surgery may be associated with a lower degree of VTE development risk compared to open surgery [13]. While a minimally invasive approach reduces abdominal wall trauma, the coagulation system activation is similar to laparotomy (LPT). Operation times for LPS procedures are frequently longer than for LPT. In addition, the use of capnoperitoneum and the reverse Trendelenburg position contributes to venous stasis, which increases VTE risk. On the other hand, patients become mobile faster after LPS.
While strong evidence for thromboprophylaxis is absent in gynaecological LPS surgery, some data suggests that the incidence of VTE increases with the difficulty of surgical procedures [14,15]. Our classification of gynaecological procedures according to VTE risk is shown in Tab. 2. Like in the case of general surgery, all patients undergoing major procedures are indicated for at least pharmacological prophylaxis. Following an extensive discussion, we also assigned scores to procedures which were classified neither as low risk (minor surgery) nor as clearly high risk. In view of weak evidence according to EBM principles, we agreed to follow the Caprini RAM which assigns 2 points for LPT and LPS procedures lasting over 45 min. For example, in young patients without any VTE RFs undergoing this type of gynaecological procedure, general measures of VTE prophylaxis are preferred to pharmacological or mechanical prophylaxis. In case additional VTE RFs – adopted from Caprini RAM, are present, pharmacological and, if needed, mechanical prophylaxis should be added. At present, we are planning a retrospective validation of the system. We have assigned 0 points to procedures lasting <45 min, because this group includes only minor surgeries (see Tab. 2) several minutes in duration, which were not classified as a VTE risk factor by a MUHP gynaecological surgeon.
2. Gynaecological procedure scoring 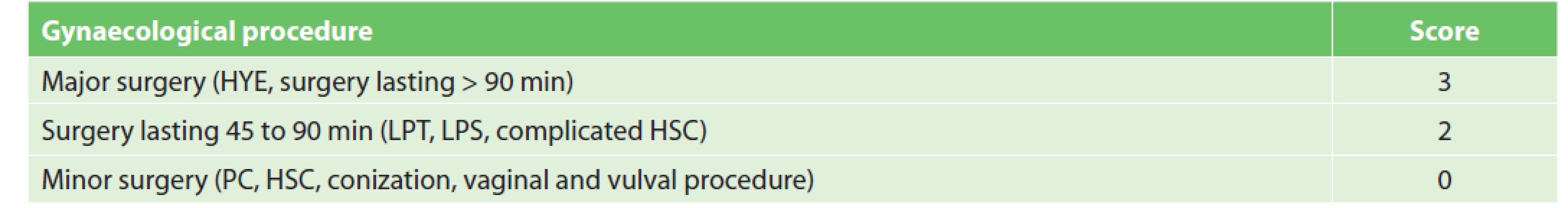
Notes: HYE – hysterectomy, LPT – laparotomy, LPS – laparoscopy, HSC – hysteroscopy, PC – probative curettage Strategies applicable to general surgery are recommended for mechanical prophylaxis as well as for the duration of pharmacological prophylaxis.
VTE prophylaxis in urology
VTE prophylaxis in urological surgery is a significant topic tackled by a range of professional organizations [1,3−5]. The Czech Urological Society recommends following guidelines published by the European Association of Urology (EAU). Despite this recommendation, we have decided to follow ACCP guidelines and Caprini RAM, primarily due to several conflicting points in EAU guidelines. The most significant discrepancies are summarized below:
■ EAU stratifies patients into three categories: low, medium, and high risk [3]. This stratification does not include some widely accepted patient-specific RFs such as congenital or acquired thrombophilia (e.g. Factor V Leiden mutation), malignancy, bed rest, sepsis, etc.
■ EAU excludes transfusion from among major bleeding criteria. Major bleeding is defined as bleeding which requires re-operation or intervention.
■ EAU also recommends the initiation of thromboprophylaxis on the day following surgery. This recommendation is based on orthopaedic surgery data which compares the timing of DOACs vs. LMWH. However, the incidence of bleeding rates and VTE in orthopaedic and urological surgery is not comparable.
■ When calculating the net benefit, the EAU assigns twice the weight to major bleeding in comparison with symptomatic VTE. On the other hand, the ACCP assigns the same weight to both complications. In effect, the EAU guidelines – which should primarily aim to prevent VTE – thus focus more on reducing the risk of bleeding complications than on reducing the incidence of thromboembolism.
■ The EAU recommends the administration of pharmacological prophylaxis four weeks after surgery in all patients.
■ The EAU admits the off-label administration of DOACs in non-standard dosing regimens (dabigatran 220 mg once daily (OD); apixaban 2.5 mg OD; edoxaban 30 mg OD or rivaroxaban 10 mg OD), despite their own statement that there is a paucity of trials to support the use of DOACs and fondaparinux in urologic surgery.
We adapted the surgical procedure classification included in the AUA (American Urological Association) Best Practice Statement and classified procedures into the following categories: open, endoscopic, laparoscopic and/or robot-assisted, anti-incontinence, pelvic reconstructive and other urological surgery procedures [4]. The stratification of procedures is provided in Tab. 3. The adopted surgical approach (laparoscopic, robotic or open) does not change the prophylactic strategy [16].
3. Urological procedure scoring 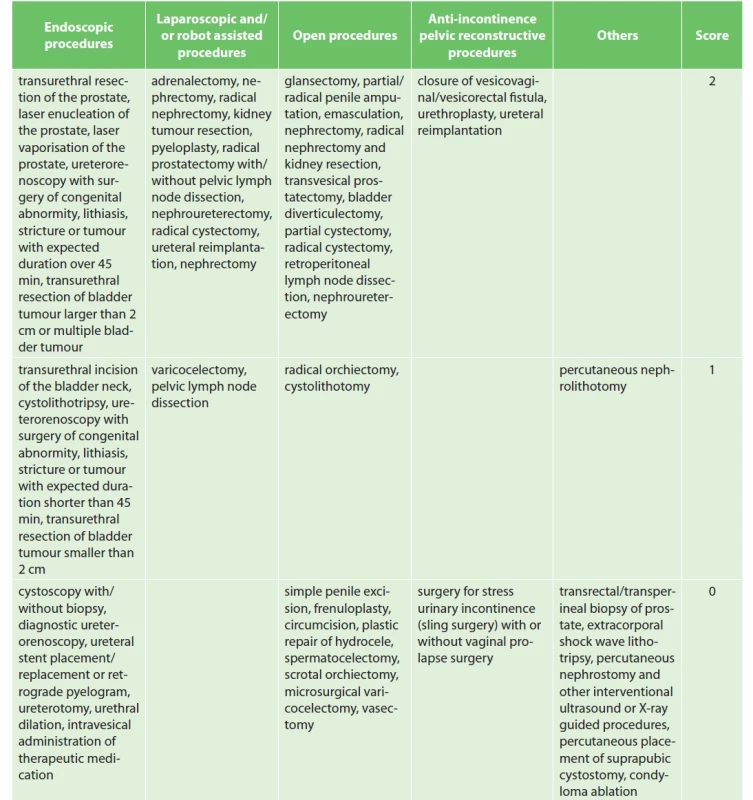
The recommended duration of pharmacological prophylaxis is 7 days. The VTE prophylaxis is extended up to 4 weeks in patients undergoing surgery for malignancy [17].
VTE prophylaxis in orthopaedics
In orthopedics, the choice of pharmacological VTE prophylaxis primarily reflects the type of surgery. For example, large orthopedic surgeries including THA (total hip arthroplasty), TKA (total knee arthroplasty) and hip fracture surgery (HFS) constitute a well-defined group supported by strong evidence. All are associated with high VTE risk, with routine pharmacological VTE prophylaxis indicated for all patients. Patient - related RFs do not affect the prophylaxis method or duration [18]. Pharmacological prophylaxis should last for 35 postoperative days in patients after THA and HFS, and 14 days after TKA. DOACs may be used in patients undergoing THA and TKA [18]. Extended prophylaxis in patients after TKA was also discussed [19]. While extended prophylaxis in these patients was not found to be more effective than in the case of THA, [20] some authors have suggesed its routine use [18]. Trials comparing the benefit of extended VTE prophylaxis after TKA and THA are performed in healthy patients with no other VTE risk factors (e.g. history of VTE, thrombofilia, myocardial infarction or stroke in the previous month, inflammatory bowel disease, acute or chronic cancer, etc.). This supported the decision to extend prophylaxis to 35 days after TKA only in patients with additional VTE RFs. RFs and scoring were adapted from the Caprini RAM [6].
Compared to strong evidence for TKA, THA and HFS, the risk of VTE in other orthopaedic surgeries has not been quite as well studied. This has led to a generally inconsistent approach to VTE prophylaxis in patients undergoing e.g. knee arthroscopy (ASK), forefoot surgery, upper limb surgery, or surgery of a fracture distal to the proximal femur. Professional society guidelines either do not specify this issue or do not recommend routine VTE prophylaxis and do not comment on the duration of pharmacological prophylaxis. Therefore, we have studied available literature in order to develop a standardized VTE scoring system and prophylactic strategy for each surgical procedure in cooperation with a surgeon.
In the new stratification system (Tab. 4) surgeries scoring 5 points are in the high VTE risk group and are indicated for extended pharmacological prophylaxis. Surgeries scoring 3 points meet the criteria of the moderate VTE risk group. Patients undergoing these procedures are indicated for pharmacological VTE prophylaxis. Surgeries scoring less than 3 points are placed into the low VTE risk group. Patients undergoing these procedures must have additional RFs to reach a total of at least 3 points (age, history of VTE, etc.) in order to be indicated for pharmacological prophylaxis. In the knee or ankle ASK, tourniquet application duration is crucial. The cut-off value was chosen by MUHP surgeons to be 60 min. If the tourniquet application lasts longer than 60 min, the patient receives pharmacological prophylaxis. If the tourniquet is applied for a shorter period of time, only patients with a total score of at least 3 points receive pharmacological prophylaxis. A similar approach was chosen for upper limb surgery. The limitation is surgery duration and the cut-off value was set to 120 min. If the surgery on the upper limb lasts longer than 120 min, the patient receives pharmacological prophylaxis. If the operation is shorter, the indication of pharmacological prophylaxis is determined by the patient’s additional RFs.
4. Orthopaedic procedure scoring 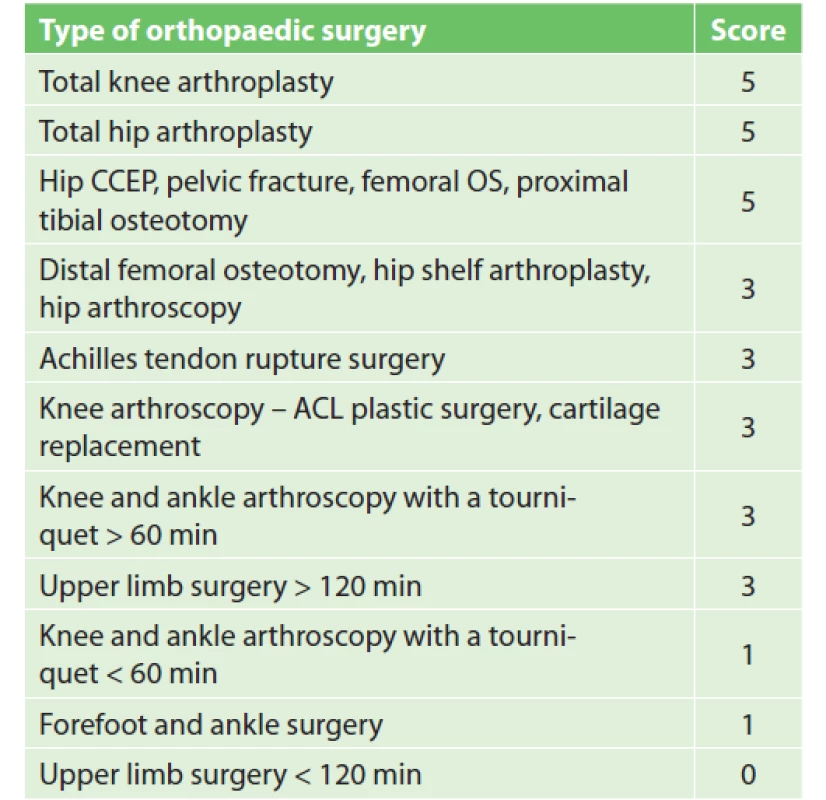
Notes: CCEP – cervical endoprosthesis, OS – osteosynthesis,
ACL – anterior cruciate ligamentIf chemical prophylaxis is indicated, it is generally recommended to last 7–10 days after surgery, or throughout the period of limited leg mobility. A different situation occurs after anterior cruciate ligament (ACL) arthroscopy, where VTE risk is higher than in other ASK procedures, mainly due to longer surgery duration and longer tourniquet application. The clinical trial showed a clear benefit of prolonging prophylaxis to 23–28 days after ASK ACL, where the frequency of deep vein thrombosis was reduced from 41% to 2.8% [21].
With the introduction of the new system, 64% of Orthopaedic Department patients met the criteria for pharmacological prophylaxis. Thus, in addition to optimizing VTE prophylaxis, the reduced consumption of LMWH and GCS – previously administered to all patients without distinction – resulted in significant financial savings. Following the implementation of the new VTE prophylaxis system, no increase in adverse events reporting VTE in outpatient follow-up was observed.
Mechanical prophylaxis in orthopaedics
Based on reviewed literature, mechanical prophylaxis administration in orthopaedics at MUHP was discontinued. Grounds include a lack of clinical trials (some with many methodological shortcomings, small patient groups, etc.), the confirmation of an insignificant clinical effect and the absence of clear recommendations for the administration of mechanical prophylaxis in patients after THA, TKA and HFS [1,22]. The ACCP suggests the use of IPC in patients after THA, TKA and HFS; however, the strength of the evidence is 2C [1]. Recommendations regarding the administration of mechanical prophylaxis in other orthopaedic procedures, except THA, TKA and HFS, are missing from available literature. The use of GCS or bandages is associated with low compliance, especially due to allday device application (with the exception of exercise time). GCS are not recommended at all for certain patient groups: patients with peripheral arterial disease, swelling of the lower limbs, skin defects (dermatitis, burns) or ulcers, etc. In addition, the application of GCS can be painful for patients after HFS. A covered limb also limits postoperative monitoring of wound healing. . Mechanical prophylaxis is preferred over chemical prophylaxis in high bleeding risk patients, where pharmacological prophylaxis is contraindicated. The risk of bleeding is re-evaluated regularly, and, following its reduction, mechanical prophylaxis is replaced by optimal pharmacological prophylaxis [1].
VTE prophylaxis in neurosurgery
Neurosurgical and spondylosurgical procedures are hazardous not only due to VTE risk, but especially due to the high risk of bleeding, which can have fatal consequences for the patient. ACCP guidelines recommend the use of mechanical prophylaxis in patients undergoing craniotomy or spinal surgery for malignancy [1]. After reducing the risk of bleeding, it is recommended to add or switch to pharmacological prophylaxis according to VTE risk. The guidelines do not specify the timing of pharmacological prophylaxis initiation. It is only mentioned that the highest risk of intracranial haemorrhage occurs 12–24 hours after the craniotomy. A review article by Pai and Douketis specifies the timing of pharmacological prophylaxis in neurosurgery [23]. Mechanical prophylaxis is best initiated before or no later than during the procedure in high bleeding risk patients. The addition of LMWH to mechanical prophylaxis or a switch from mechanical to pharmacological prophylaxis is recommended within 48–72 hours after the procedure once hemostasis is partially restored.
Following a discussion with surgeons, the following preventive measures were introduced at the Neurosurgical Department of MUHP. The majority of neurosurgical procedures fall into the moderate or high VTE risk group (see Tab. 5), i.e. pharmacological prophylaxis should be administered. In all patients undergoing craniotomy or spinal surgery, mechanical prophylaxis is started before the operation and continues throughout the entire surgery. Mechanical prophylaxis is replaced by pharmacological prophylaxis in moderate VTE risk patients (3–4 points). Patients in the high VTE risk group (5+ points) should receive both mechanical and pharmacological prophylaxis. Pharmacological prophylaxis begins 12–72 hours after the procedure, in view of the risk of bleeding and according to the surgeon’s assessment and postoperative computed tomography (CT) or magnetic resonance imaging (MRI) follow-up. In the case of a serious bleeding complication, only mechanical prophylaxis is recommended. Acute brain injury, including intracranial haemorrhage, also increases the risk of coagulopathy, i.e. pharmacological prophylaxis should thus begin as soon as possible [24].
5. Neurosurgical procedure scoring 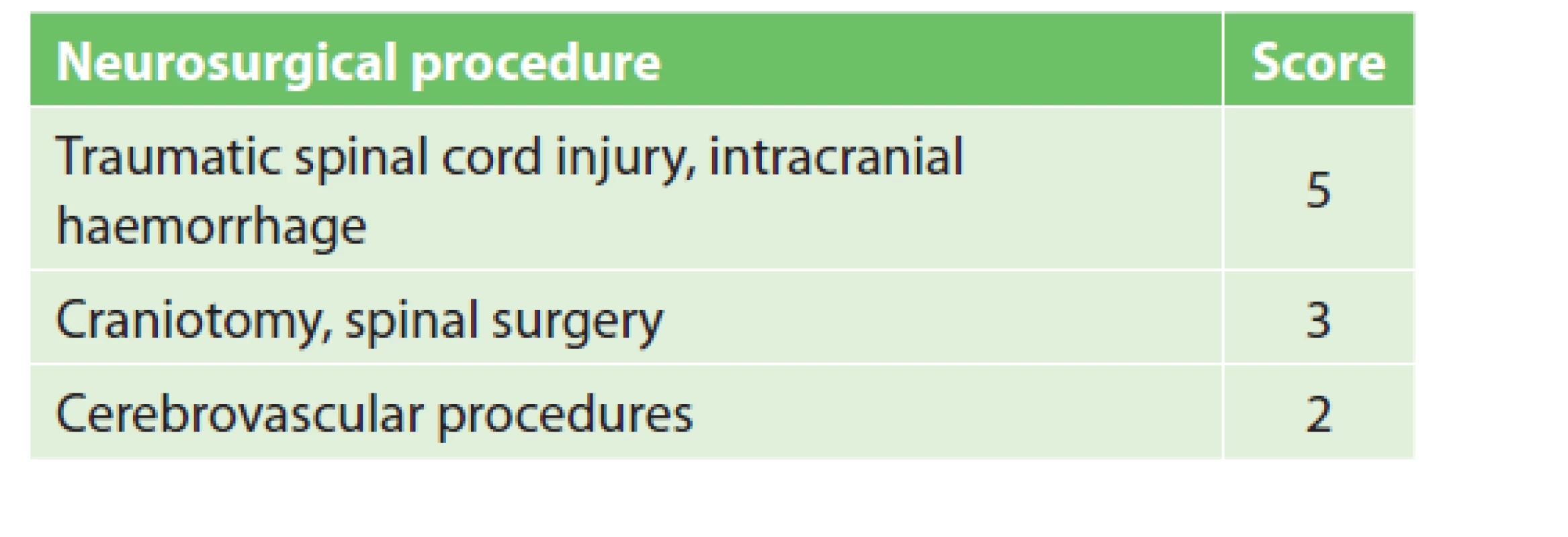
In the case of cerebrovascular procedures (carotid endarterectomy, CEA; carotid artery angioplasty with stenting, CAS) it was necessary to critically assess the approach to VTE prophylaxis, as this case is not described in any available guidelines. Patients scheduled for CEA use antiplatelet monotherapy (low dose aspirin, clopidogrel) or dual antiplatelet therapy (DAPT), with the latter preferable in the case of a recent acute ischemic stroke or transient ischemic attack (TIA). All CAS patients have dual antiplatelet therapy [25]. The routine administration of pharmacological prophylaxis would increase the risk of bleeding in patients on DAPT. After evaluating the risks and benefits of pharmacological vs mechanical prophylaxis, a consensus with neurosurgeons was reached: all patients are started on mechanical prophylaxis on admission to hospital. In patients on antiplatelet monotherapy after CEA, pharmacological prophylaxis begins 12 hours after the procedure. In case a haemorrhagic complication occurs, pharmacological prophylaxis is contraindicated. Patients on DAPT undergoing CEA receive only mechanical prophylaxis. The co-administration of DAPT and pharmacological prophylaxis is not used in the Neurosurgical Department due to the high risk of fatal bleeding complications. In case DAPT is ended after CEA and only one antiplatelet drug remains in therapy, pharmacological prophylaxis can be initiated. In patients undergoing CAS only mechanical prophylaxis is provided due to the need of DAPT administration after the procedure.
Although it has not been validated for neurosurgery, the VTE RFs scoring system was adapted from the Caprini RAM [6]. We adjusted the scoring system by adding two more risk factors: recent TIA (within the last month) and corticosteroid therapy (each worth 1 point). The former was added because patients undergoing treatment for carotid stenosis (CEA or CAS) often have a history of TIA. The latter was added since dexamethasone prophylaxis is used to prevent oedema in patients with brain or spinal cord tumours. The duration of corticosteroid therapy after a procedure varies and depends on the clinical condition and post-operative CT/MRI. Glucocorticoid administration is associated with a higher risk of VTE [26].
Recommended VTE prophylaxis duration is 7–10 days or until a patient becomes fully ambulatory. In patients undergoing extensive procedures for malignancy, VTE prophylaxis should be extended up to 4 weeks. In patients with spinal lesions, pharmacological prophylaxis should last until the end of physical therapy.
VTE prophylaxis in eye surgery
Ocular procedures are generally considered to be low risk with respect to both VTE and perioperative bleeding. Since the majority of procedures performed at the Ophthalmological Department last less than one hour, patients are able to ambulate easily after surgery and are discharged in the evening on the day of surgery. As a result, pharmacological and mechanical VTE prophylaxis is not needed. In the past, all patients received mechanical prophylaxis, but this was eliminated with the implementation of the new system. The new VTE prophylaxis strategy consists of early ambulation and optimal hydration.
Pharmacoprophylaxis in special populations Renal impairment
When dosing DOAC or LMWH in patients with kidney disease (glomerular filtration below 30 ml/min) we recommend an individual approach and consultations with a clinical pharmacist. It is possible to adjust the dose of LMWH or DOACs based on Anti-Xa activity or plasmatic level monitoring. A further option is the use of UFH, where no dosage adjustment is required.
Obese patients
Whether or not an increase in LMWH dosage in obese patients is suitable has been discussed by experts for many years. Due to the pharmacokinetic properties of LMWH (hydrophilicity) and the distribution of these drugs in obese patients (water content in adipose tissue, etc.), a dose increase seems rational. However, there is still a paucity of randomised clinical trials (RCT) and an absence of guidelines capable of recommending a universal approach [1,2]. The evidence is based primarily on bariatric surgery studies [27,28]. A majority of existing studies suggests an increase in LMWH dosage by 30% in patients with BMI ≥40 kg/m2 while some recommend dividing the total daily dose into two doses, e.g. to administer enoxaparine 40 mg twice daily [23,29,30]. We have abandoned the idea of twice daily administration due to the high risk of confusion with the therapeutic dosing regimen and possible medication errors. With regard to the information above, we have decided to follow a specially developed protocol (see Tab. 6). Our dosing regimen is off-label. However, as noted above, the dosing regimen is evidence-based, meeting the criteria for effective pharmacological VTE prophylaxis, as required by the relevant authorities. To verify the efficacy of LMWH, Anti-Xa activity can be monitored. DOAC dose adjustment is consulted individually.
6. LMWH doses in obese patients 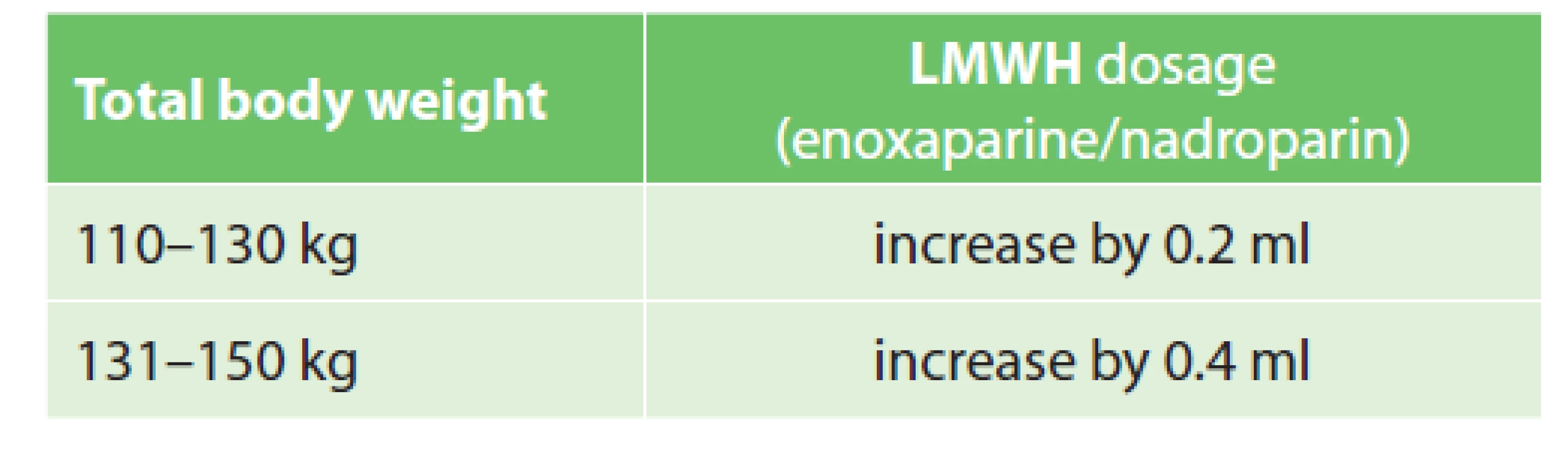
CONCLUSION
The implementation of the VTE prophylaxis system resulted in a uniform and more rational prescription of pharmacological prophylaxis as well as in financial savings. The development of the app increased compliance with the system and, in combination with real-time auditing, significantly improved safe drug administration practices at MUHP. The resulting system, which is based on multidisciplinary cooperation between a physician, a clinical pharmacist and an IT specialist, is unique in continental Europe and contains wide range of surgical procedures and matching VTE prophylaxis, which has never before been published in this scope. It demonstrates the contribution of clinical pharmacists to the improvement of hospital care quality.
Our system tackles the general absence of guidelines which would cover the full range of surgeries and technical procedures. Strict exclusion criteria (e.g. patients at high VTE risk, anticoagulated patients, with a history of VTE, morbidly obese, with renal impairment) in many RCTs likewise do not correspond to routine clinical practice. This highlights the importance of the role of a clinical pharmacist and indicates that his/her ability to critically evaluate and prioritize each individual patient’s needs over simple compliance with the guidelines is truly essential.
The MUHP system is regularly tested during the course of providing clinical pharmaceutical care to patients at surgical departments. With the technological opportunities provided by the innovated app, we would like to subject the “blank spaces” of the system to thorough validation in the future (e.g. orthopaedic procedures on the upper limb and ASK of the knee joint, etc.) and provide high-quality pharmacoeconomic data.
The presented article is unique in its complexity with respect to the number of developed VTE prophylaxis approaches in various surgery types. In this respect, the new system may also serve as a source of inspiration for other clinical pharmacy departments and surgery departments, as the majority of surgical professional societies currently lack a clear methodology in this area.
Acknowledgements
We are very grateful to the surgeons of Military University Hospital Prague: Milan Čermák from the Urological Department, David Netuka from the Neurosurgical Department, Pavla Svobodová from the Gynaecological Department, Martina Svobodová and Martin Šín from the Ophthalmological Department, Michal Tuček from the Orthopaedic Department and Pavel Záruba from the Surgical Department and Zbygniew Kičerka from the IT Department for their cooperation in setting up the VTE prophylaxis system.
Conflict of interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal except congress abstracts and clinical guidelines.
PharmDr. Irena Murínová
Department of Clinical Pharmacy,
Military University Hospital Prague
U Vojenské nemocnice 1200
169 02 Prague
e-mail: irena.murinova@uvn.cz
ORCID: 0000-0003-2914-1037
Sources
1. Gould M, Garcia D, Wren S, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012 feb;141 (2 Suppl):e227S-e277S. doi: 10.1378/ chest.11-2297.
2. Samama C, Afshari A, Force, ESA VTE Guidelines task. European guidelines on perioperative venous thromboembolism prophylaxis. Eur J Anaesthesiol. 2018 Feb;35(2):73−153. doi: 10.1097/ EJA.0000000000000702.
3. Tikkinen K, Cartwright R, Gould M, et al. Thromboprophylaxis. [Online]. Available at: https://uroweb.org/guideline/ thromboprophylaxis/ Office, EAU Guidelines, Arnhem, 2020. [Accessed 17 Jun 2020].
4. Forrest J, Clemens J, Finamore P, et al. AUA best practice statement for the prevention of deep vein thrombosis in patients undergoing urologic surgery. J Urol. 2009 March;181(3):1170−1177. doi: 10.1016/j.juro.2008.12.027.
5. NICE guideline, Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. Published 2018 March;[Online]. Available at: https:// www.nice.org.uk/guidance/ng89.
6. Caprini J. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005 Feb-Mar;51(2-3):70−78, 2005 Feb-Mar. doi: 10.1016/j.disamonth. 2005.02.003.
7. Lobastov K, Barinov V, Schastlivtsev I, et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016 Apr;4(2):153−160. doi: 10.1016/j. jvsv.2015.09.004.
8. Obi A, Pannucci C, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically Ill surgical patients. JAMA Surg. 2015 Oct;150(10):941−948. doi: 10.1001/ jamasurg.2015.1841.
9. Cronin M, Dengler N, Krauss E, et al. Completion of the update Caprini risk assessment model (2013 Version). Clin Appl Thromb Hemost. 2019 Jan-Dec;25 : 1−10. doi: 10.1177/1076029619838052.
10. Anderson D, Morgano G, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019 Dec;3(23):3898−3944. doi: 10.1182/bloodadvances. 2019000975.
11. State Institute for drug control, medicinal products database, summary of product characteristics [Online]. Available at: https://www.sukl.eu/modules/medication/ search.php [Accessed 28 Apr 2020].
12. Bailey C, Ahuja M, Bartholomew K, et al. Guidelines for day-case surgery 2019: Guidelines from the association of anaesthetists and the British Association of Day Surgery. Anaesthesia 2019, Apr. 8;74(6):778−792. doi: 10.1111/ anae.14639. Epub 2019 Apr 8.
13. Mann W. Overview of preoperative evaluation and preparation for gynecologic surgery 2020. Topic 3301 Version 59.0. UpToDat. This topic last updated: Jul 27, 2021.
14. Nick A, Schmeler K, Frumovitz M, et al. Risk of thromboembolic disease in patients undergoing laparoscopic gynecologic surgery. Obstet Gynecol. 2010 Oct;116(4):956−961. doi: 10.1097/ AOG.0b013e3181f240f7.
15. Jorgensen E, Li A, Modest A, et al. Incidence of venous thromboembolism after different modes of gynecologic surgery. Obstet Gynecol. 2018 Nov;132(5):1275−1284. doi: 10.1097/ AOG.0000000000002918.
16. Violette P, Cartwright R, Briel M, et al. Guideline of guidelines: thromboprophylaxis for urological surgery. BJU Int. 2016 Sep;118 : 351−358. doi: 10.1111/ bju.13496. Epub 2016 Apr 29.
17. Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-associated venous thromboembolic disease, Version 1.2015. J Natl Compr Canc Netw. 2015 Sep;13(9):1079−1095. doi: 10.6004/jnccn.2015.0133.
18. Falck-Ytter Y, Francis C, Johanson N, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis. 9th ed: American College of Chest Physicians Evidence - Based Clinical Practice Guidelines. Chest 2012 Feb;141(2) Suppl:e278S − e325S. doi: 10.1378/chest.11-2404.
19. Warwick D, Friedman R, Agnelli G, et al. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br. 2007 Jun;89(6):799−807. doi: 10.1302/0301-620X.89B6.18844.
20. Comp P, Spiro T, Friedman R, et al. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. J Bone Joint Surg Am. 2001 Mar;83(3):336−345. doi: 10.2106/00004623-200103000-00004.
21. Marlovits S, Striessnig G, Schuster R, et al. Extended-duration thromboprophylaxis with enoxaparin after arthroscopic surgery of the anterior cruciate ligament: a prospective, randomized, placebo - controlled study. Arthroscopy 2007 Jul;23(7):696−702. doi: 10.1016/j.arthro. 2007.02.001.
22. Kinov P, Tenchev PP, Ellis M, et al. Antithrombotic prophylaxis in major orthopaedic surgery: an historical overview and update of current recommendations. Int Orthop. 2014 Jan;38(1):169−175. doi: 10.1007/s00264-013-2134-8. Epub 2013 Oct 11.
23. Pai M, Douketis J. Prevention of venous thromboembolic disease in adult nonorthopedic surgical patients. UpToDate 2019. Topic 1339 Version 100.0. This topic last updated: Apr 09, 2021.
24. Rajajee V. Management of acute moderate and severe traumatic brain injury. UpToDate 2019. Topic 4826 Version 31.0. This topic last updated: May 22, 2021.
25. Massop D. Dave R, Metzger C, et al. Stenting and angioplasty with protection in patients at high‐risk for endarterectomy: SAPPHIRE Worldwide Registry first 2,001 patients. Catheter Cardiovasc Interv. 2009 Feb;73(2)129−136. doi: 10.1002/ ccd.21844.
26. Johannesdottir S, Horváth-Puhó E, Dekkers O, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013 May 13;173(9):743−752. doi: 10.1001/jamainternmed. 2013.122.
27. Hamad G, Bariatric surgery: Postoperative and long-term management of the uncomplicated patient. 2019. Topic 88610 Version 14.0. UpToDate. This topic last updated: May 04, 2021.
28. Mechanick J, Youdim A, Jones D, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013 Mar;21,Suppl 1:S1−S27. doi: 10.1002/ oby.20461.
29. Parker S, McGlone E, Knight W, et al. Enoxaparin venous thromboembolism prophylaxis in bariatric surgery: A best evidence topic. Int J Surg. 2015 Nov;23 : 52−56. doi: 10.1016/j.ijsu.2015.09.005. Epub 2015 Sep 21.
30. Al Otaib N, Bootah Z, Al Ammari M, et al. Assessment of anti-factor Xa activity of enoxaparin for venous thromboembolism prophylaxis in morbidly obese surgical patients. Ann Thorac Med. 2017 Jul-Sep;12(3):199−203. doi: 10.4103/atm. ATM_31_17.
Labels
Surgery Orthopaedics Trauma surgery
Article was published inPerspectives in Surgery

2021 Issue 12-
All articles in this issue
- Střípky současnosti
- Retroperitoneal hematoma: diagnosis and treatment
- Video-assisted and robotic-assisted thoracoscopic pulmonary lobectomies, our experience
- Laparoscopic versus open elective right hemicolectomy with curative intent for colon adenocarcinoma
- Transhepatic perforation of the gallbladder with massive intraperitoneal hemorrhage as a rare complication of acute cholecystitis
- Spontaneous retroperitoneal bleeding in COVID-19 patients – two case reports
- Tumor mimicking gastric ulcer penetrating asymptomatically into the pancreas
- Blank spaces in the management of venous thromboembolism prophylaxis at surgical departments and the impact of a clinical pharmacist
- Perspectives in Surgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Retroperitoneal hematoma: diagnosis and treatment
- Laparoscopic versus open elective right hemicolectomy with curative intent for colon adenocarcinoma
- Video-assisted and robotic-assisted thoracoscopic pulmonary lobectomies, our experience
- Tumor mimicking gastric ulcer penetrating asymptomatically into the pancreas
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career