-
Medical journals
- Career
Pancreaticoduodenectomy for pancreatic cancer in elderly patients – a single-centre experience
Authors: A. Nikov; P. Záruba; F. Bělina; M. Ryska
Authors‘ workplace: Surgery department, 2nd Faculty of medicine Charles University and Central Military Hospital, Prague
Published in: Rozhl. Chir., 2018, roč. 97, č. 1, s. 34-38.
Category: Original articles
Overview
Introduction:
Conflicting results can be found in the literature with regards to the impact of age on mortality and morbidity associated with pancreaticoduodenectomy. Insufficient methodological quality is a problem in most undertaken studies. Although rather few papers have focused on whether age has any impact on long-term survival after pancreaticoduodenectomy, their conclusions agree – i.e. higher age is not associated with shorter survival after pancreaticoduodenectomy. The aim of this paper is to compare short - and long-term outcomes of pancreaticoduodenectomy for pancreatic cancer in individual age groups.Method:
Retrospective comparative analysis of data obtained from medical records of patients after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma at a single centre, in the period 01/2011−12/2015. Differences in morbidity, serious complications (equal to or higher than grade III according to the Clavien-Dindo classification), the incidence of postoperative pancreatic fistula and the probability of completing adjuvant chemotherapy were tested using the chi-square test and Fisher’s exact test, as appropriate, in patient age categories <65 years, 65–75 years and ≥75 years. Long-term survival rates in the individual age categories were evaluated using Kaplan-Meier curves and the long-rank test. The significance level was defined as α = 0.05.Results:
The 30-day mortality in patient age categories <65, 65−75 and ≥75 years was 2.22%, 2.78% and 7.69%, respectively (p = 0.60), and the 90-day mortality was 6.66%, 5.56% and 7.69%, respectively (p=0.94). Complication rates grade III and higher according to the Clavien-Dindo classification system were 33.3%, 27.8% and 23.1%, respectively (p=0.73). The estimated median survival was 19.2, 21.1 and 11.8 months (p = 0.26). Complete adjuvant chemotherapy was administered to 84.4%; 55.6% and 15.4% of the patients − depending on the age group (p<0.001).Conclusion:
Both short - and long-term outcomes after pancreaticoduodenectomy in selected patients with resectable pancreatic cancer older than 75 years are not significantly different from those achieved in the younger age groups. Thus, the age per se should not represent a contraindication for pancreaticoduodenectomy or adjuvant chemotherapy in patients with pancreatic cancer.Key words:
pancreaticoduodenectomy −pancreatic cancer − elderly patientsINTRODUCTION
Pancreaticoduodenectomy (PDE) is among procedures with a relatively high morbidity and risk of mortality. Age is therefore one of the intuitive selection criteria used when indicating a patient for this procedure. The influence of age on the mortality and morbidity of pancreatic resections has been addressed in a number of retrospective studies and their meta-analyses. Some of them came to the conclusion that older age is not a factor associated with higher mortality and morbidity [1−6], others came to the opposite conclusion [7−9]. One meta-analysis exists with a “compromising” conclusion, that the group of patients over 80 years of age has a higher mortality and morbidity after pancreatic resections, with the exception of those patients, which have an index of comorbidity comparable to those aged younger than 80 years [10].
Due to discrepancies in the available data and their methodological inconsistencies, we decided to retrospectively evaluate the effect of age on mortality, morbidity and long-term survival in our own homogenous set of patients undergoing pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head.
METHOD
We performed a retrospective comparative analysis of data available from patient medical records who underwent pancreaticoduodenectomy at the Surgery Department at the Second Faculty of Medicine, Charles University and Central Military Hospital between 01/2011−12/2015 with histopathological findings of ductal adenocarcinoma of the pancreas in the resected specimen. The studied parameters were: age at the time of surgery, 30-day mortality, 90-day mortality, severe postoperative complications (Grade III or higher according to the Clavien-Dindo classification [11]), incidence of clinically significant postoperative pancreatic fistula (grade B and C, defined by ISGPF [12]), completion of adjuvant chemotherapy and the reason for possibly not administering adjuvant chemotherapy. Long-term survival was evaluated using the estimated median survival time in months with a 95% confidence interval – CI; estimated 5-year overall survival and hazard ratio – HR. For statistical evaluation of the effect of age on the studied parameters, the patients were divided into 3 age groups: <65 years, 65−75 years and ≥75 years. Categorical variables (morbidity, mortality, risk of postoperative pancreatic fistula (POPF), extent of completed adjuvant chemotherapy and reasons for non-administration of adjuvant chemotherapy) were compared within the age groups using the Chi-square test and Fisher´s exact test – based on appropriateness. Reasons for not administering adjuvant chemotherapy were, for statistical processing needs, divided into 2 groups: reasons justified by the patient’s medical condition (postoperative complications, worsening of performance status) and unjustified reasons (treatment was not indicated by the oncologist without naming the reason, or due to age, or refusal by the patient). The Kaplan-Meier method and log-rank test were used to compare long-term survival. Patients surviving up to the date of statistical processing (April 30, 2017) were censored. The level of statistically significant difference was set at α = 0.05. Statistical processing was performed using the software MedCalc, version 17.6. (Ostende, Belgium). Consent with the processing of data from medical records was included in the informed consent regarding performance of the surgery; their retrospective evaluation therefore did not require consent by the ethical committee and was not in conflict with the principles of the Declaration of Helsinki.
RESULTS
Between 01/2011−12/2015, we performed 94 PDE’s for ductal adenocarcinoma of the head of the pancreas, the average age of the operated patients was 64 years (range 32−82 years). The overall 30-day mortality was 3.2 % (3/94 pts.), 90-day mortality 6.4% (6/94 pts.), frequency of Clavien-Dindo complications ≥ grade III was 29.8% (28/94 pts.), frequency of clinically significant POPF was 13.8% (13/94 pts.), estimated 5-year survival rate was 22.2%, estimated median survival time was 19.4 months (95% CI 17.4–29.5 m.), 63.8% (60/94 pts.) underwent complete adjuvant chemotherapy. The group of patients under 65 years of age represented 47.8% of the operated patients (average age 56 years), the group of patients aged 65−75 years 38.3% of the operated patients and the group of patients over 75 years of age represented only 13.9% of the operated patients, with an average age of 78 years and a maximum age of 82 years. Differences in mortality, morbidity and long-term survival between the individual age groups did not reach statistical significance – Tab. 1, Graph 1 and Tab. 2. The only statistically significant difference observed between the age groups was the probability of completing adjuvant chemotherapy – Tab. 1. Reasons for non-administration of adjuvant chemotherapy are listed in Graph 2. The representation of unjustified reasons for non-administration of chemotherapy (not indicated by the oncologist without naming the reason, due to age, patient refusal) increased with age; however, the difference between the groups was not statistically significant (43%, 56%, 73% in the groups under 65 yrs., 65−75 yrs., over 75 yrs., resp.; p=0,2057).
1. Short- and long-term outcomes in individual age groups 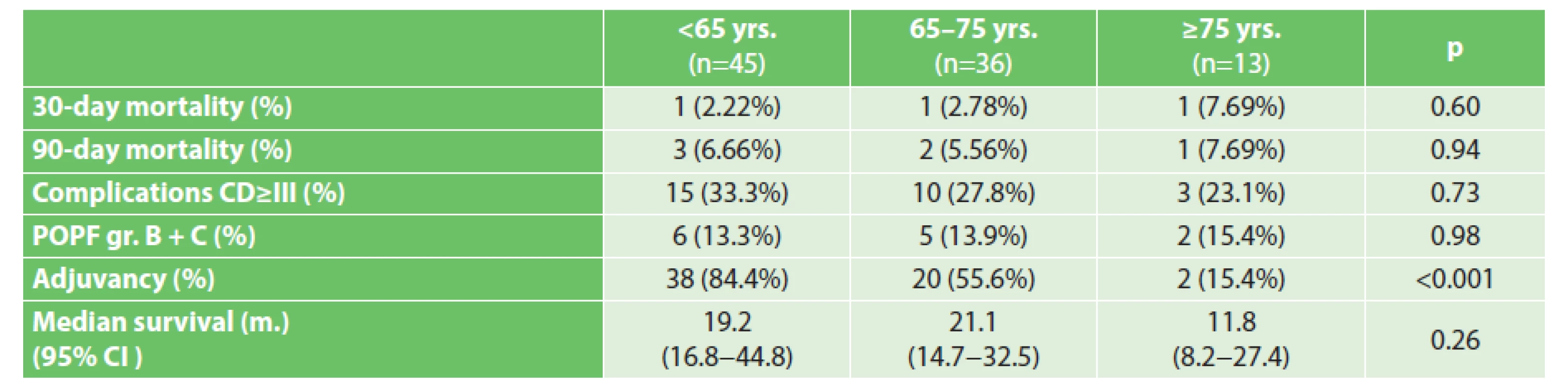
2. Hazard ratios of death (HR with 95% CI) between age groups – columns/rows 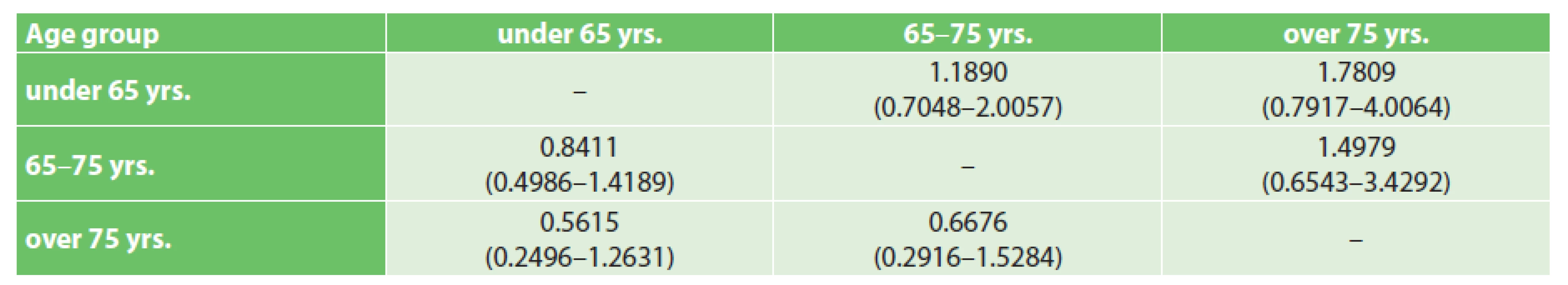
Note: P = 0.2589 1. Kaplan-Meier survival curves according to age 
2. Causes of non-administration of adjuvant chemotherapy 
DISCUSSION
Although, according to published data regarding patients with pancreatic cancer over 80 years of age, the benefit of surgical resection for prolonging overall survival (in comparison with chemotherapy alone) is smaller than in younger age groups, a difference in OS between operated and non-operated patients exists and is statistically significant [13]. It is therefore incorrect to limit access to radical surgical treatment to patients based solely on their age, especially since pancreatic cancer is one of the “safer” indications for PDE. Prolonging OS is not the only rational aim when considering PDE in seniors with good biological status. The quality of life is statistically significantly better in patients undergoing radical surgical resection than in patients treated palliatively [14]; surgical treatment is the only treatment modality that independently increases the quality of life compared to the condition of the patient prior to the beginning of treatment [15].
The greatest methodological hurdle when incorporating the conclusions of these studies and those mentioned in the introduction into practice is the heterogeneity of the evaluated groups, especially in the case of mixed patient sets which consist of all types of periampullary tumours, or various types of resection procedures on the pancreas. Among periampullary tumours, carcinoma of the distal common bile duct is known to be associated with the greatest risk of pancreatic fistula after PDE, carcinoma of the head of the pancreas is associated with the lowest risk [16]. Because the majority of deaths after PDE are associated with postoperative pancreatic fistula (POPF), the differing risk of POPF depending on the indication also projects onto mortality – the mortality of PDE for pancreatic cancer consistently presented in the literature in high-volume centres is less than 5%, the mortality of PDE for remaining periampullary tumours is 8.9% [17]. The reason for this difference is the “soft” consistency of the parenchyma of the pancreas in most patients with non-pancreatic malignancies, whereas long-term ductal obstruction by cancer of the pancreatic head induces chronic pancreatitis in the remainder of the gland, which reduces the risk of POPF. Older age and its associated comorbidities do not limit the capability of managing the surgery itself and an uncomplicated postoperative course, but mainly limit the capacity to handle protracted septic complications associated with POPF. Therefore studies have emerged, which evaluate the effect of age on the mortality of PDE based on the indication for PDE [18]. Pancreatic cancer and chronic pancreatitis are thus considered “safe” indications for PDE in patients over 80 years of age; contrarily, carcinoma of the distal common bile duct, carcinoma of the ampulla of Vater, and neuroendocrine tumours are considered “risky” indications.
Significantly less studies address the effect of age on long-term survival of patients undergoing curative surgical treatment. They agree that age does not have a significant impact on long-term survival of operated patients [3,19,20]. It may thus be assumed, that contrarily to the general population, where the natural probability of death increases with age and older age is associated with a shorter life-expectancy [21], survival of patients with pancreatic cancer is determined by unfavourable biological characteristics of the tumour, not age.
Although the maximum incidence of pancreatic cancer in the Czech Republic is observed in the 8th decade and patients over 75 years of age represent 27.4% of pancreatic cancers diagnosed in stages I and II (AJCC 8th ed.) [22], patients over 75 years of age represent only 13.9% of the patients operated on at the authors’ workplace. Almost half of the operated patients are under the age of 65, despite that the data from ÚZIS report that these patients represent only 36.7% of carcinomas in stages I and II [22]. The reality that the distribution of the age groups of the operated patients does not copy the distribution of the incidence of pancreatic cancer corresponds to the preference of surgical treatment by younger patients and the selection of elderly patients.
The analysed data from the authors’ workplace lead to the conclusion that in a selected population of elderly patients, the same results of surgical treatment may be attained as in the group of younger patients. Although it seems that there is a marked difference in the estimated median of survival of the oldest group compared to the other groups, according to the methodologically correct testing of the null hypothesis (log-rank test), it is not statistically significant. It cannot be stated, that the discovered difference between the groups is large enough to be considered statistically significant. Perhaps better than the comparison of medians of survival, long-terms results can be characterised by the hazard ratio (HR) of death during the course of the studied interval, which quantifies the „divergence“ of the survival curves on the Kaplan-Meier graph. Tab. 2 presents the HR (hazard ratios) with 95% confidence intervals, which overlap significantly – this may only be interpreted that there is no statistically significant difference in the risk of death during the course of the studied interval between the study groups. This observation is in accordance with the literature cited in the introduction. Selection criteria useful in practice for the selection of elderly patients suitable for pancreaticoduodenectomy are appearing in the literature in the form of various functional tests (timed up-and-go test – TUG [23], 6 minute walk distance [24] etc.) or morphological parameters (cross-sectional area of the psoas muscle or psoas muscle volume [25]), which evaluate the „frailty“ of the elderly patient. Complex assessment by a specialized geriatrician is the best way to maintain balance between the chance at radical treatment and risk of PDE in the elderly patient [26]. It may be assumed, that by using more sophisticated methods for the selection of elderly patients, more patients over 75 years of age in clinical stages I and II may be selected than the current theoretical half (13.9% of operated patients at the authors’ workplace - of the total number of resectable carcinomas – 27.4%).
It is also important to note that patients over 75 years of age have a significantly lower chance of receiving adjuvant chemotherapy than younger patients, although they do not have a significantly higher postoperative morbidity, which would objectively hinder the administration of adjuvant therapy (Graph 2). Only 15.4% of our patients over 75 years of age received adjuvant therapy, while in the younger groups 84.4% and 55.6% of patients were administered adjuvant therapy (p<0,001), despite the fact that the prognostic benefit of adjuvant chemotherapy has been established in several RCTs [27−29]. This observation is also in accordance with the published literature [30]. The selection of elderly patients for adjuvant chemotherapy therefore seems to present the greatest unresolved issue in the care of these patients.
CONCLUSION
From the analysis of patients operated on at the authors‘ workplace, it can be stated, in concordance with other published data, that with proper patient selection, no statistically significant difference in morbidity, mortality and long-term survival after PDE for adenocarcinoma of the pancreas in patients over 75 years of age compared to younger patients was observed. Older age per se should therefore not be an automatic contraindication for PDE in patients with resectable pancreatic cancer or contraindication for adjuvant treatment.
Supported by the institutional support of ÚVN MO1012
Conflict of interest
The authors of the article declare, that there is no conflict of interest associated with the creation of this article and that this article was not published in any other journal.
MUDr. Andrej Nikov
Surgery department, 2nd Faculty of medicine
Charles University and Central Military Hospital, Prague
U vojenské nemocnice 1200/1
169 02 Praha
e-mail: andrej.nikov@gmail.com
Sources
1. Paiella S, De Pastena M, Pollini T, et al. Pancreaticoduodenectomy in patients >/= 75 years of age: Are there any differences with other age ranges in oncological and surgical outcomes? Results from a tertiary referral center. World J Gastroenterol 2017;23 : 3077−83.
2. Liang DH, Shirkey BA, Rosenberg WR, et al. Clinical outcomes of pancreaticoduodenectomy in octogenarians: a surgeon experience from 2007 to 2015. J Gastrointest Oncol 2016;7 : 540−6.
3. Turrini O, Paye F, Bachellier P, et al. Pancreatectomy for adenocarcinoma in elderly patients: postoperative outcomes and long term results: a study of the French Surgical Association. Eur J Surg Oncol 2013;39 : 171−8.
4. Shiozawa S, Usui T, Kuhara K, et al. Eligibility criteria specific to pancreaticoduodenectomy for octogenarians: Single-center opinion. Anticancer Res 2017;37 : 2037−43.
5. Oliverius M, Kala Z, Varga M, et al. Radical surgery for pancreatic malignancy in the elderly. Pancreatology 2010;10 : 499−502.
6. Makary MA, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg 2006;10 : 347−56.
7. Kim SY, Weinberg L, Christophi C, et al. The outcomes of the pancreaticoduodenectomy in patients aged 80 or older. A systematic review and meta-analysis. HPB (Oxford) 2017;19 : 475−82.
8. Sukharamwala P, Thoens J, Szuchmacher M, et al. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: a meta-analysis and systematic review. HPB (Oxford) 2012;14 : 649−57.
9. Sperti C, Moletta L, Pozza G. Pancreatic resection in very elderly patients: A critical analysis of existing evidence. World J Gastrointest Oncol 2017;9 : 30−6.
10. Casadei R, Ricci C, Lazzarini E, et al. Pancreatic resection in patients 80 years or older: a meta-analysis and systematic review. Pancreas 2014;43 : 1208−18.
11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240 : 205−13.
12. Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138 : 8−13.
13. Marmor S, Burke EE, Virnig BA, et al. A comparative analysis of survival outcomes between pancreatectomy and chemotherapy for elderly patients with adenocarcinoma of the pancreas. Cancer 2016;122 : 3378−85.
14. Ryska M, Dusek L, Pohnan R, et al. Quality of life is an important factor in the indication in patients with advanced pancreatic carcinoma--a prospective multicentric study. Rozhl Chir 2012;91 : 199−208.
15. Crippa S, Dominguez I, Rodriguez JR, et al. Quality of life in pancreatic cancer: analysis by stage and treatment. J Gastrointest Surg 2008;12 : 783−93; discussion 93−4.
16. Kleespies A, Albertsmeier M, Obeidat F, et al. The challenge of pancreatic anastomosis. Langenbecks Arch Surg 2008;393 : 459−71.
17. Bourgouin S, Ewald J, Mancini J, et al. Predictors of survival in ampullary, bile duct and duodenal cancers following pancreaticoduodenectomy: a 10-year multicentre analysis. J Gastrointest Surg 2015;19 : 1247−55.
18. Bergquist JR, Shubert CR, Ubl DS, et al. Risk by indication for pancreaticoduodenectomy in patients 80 years and older: a study from the American College of Surgeons National Surgical Quality Improvement Program. Hpb 2016;18 : 900−7.
19. Melis M, Marcon F, Masi A, et al. The safety of a pancreaticoduodenectomy in patients older than 80 years: risk vs. benefits. HPB (Oxford) 2012;14 : 583−8.
20. Renz BW, Khalil PN, Mikhailov M, et al. Pancreaticoduodenectomy for adenocarcinoma of the pancreatic head is justified in elderly patients: A retrospective cohort study. Int J Surg 2016;28 : 118−25.
21. Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet 2009;374 : 1196−208.
22. Dušek L, Kubásek M, Koptíková J et al. Epidemiologie zhoubných nádorů v České republice. Masarykova univerzita 2005. Available from: http://www.svod.cz (Verze 7.0 [2007], ISSN 1802 – 8861.)
23. Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: A multicenter prospective cohort study. Eur J Surg Oncol 2015;41 : 844−51.
24. Hayashi K, Yokoyama Y, Nakajima H, et al. Preoperative 6-minute walk distance accurately predicts postoperative complications after operations for hepato-pancreato-biliary cancer. Surgery 2017;161 : 525−32.
25. Amini N, Spolverato G, Gupta R, et al. Impact total psoas volume on short - and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 2015;19 : 1593−602.
26. Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg 2014;259 : 960−5.
27. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310 : 1473−81.
28. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350 : 1200−10.
29. Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304 : 1073−81.
30. Nagrial AM, Chang DK, Nguyen NQ, et al. Adjuvant chemotherapy in elderly patients with pancreatic cancer. Br J Cancer 2014;110 : 313−9.
Labels
Surgery Orthopaedics Trauma surgery
Article was published inPerspectives in Surgery

2018 Issue 1-
All articles in this issue
- Plastic surgery of advanced age
- Breast cancer in elderly patients over 70 years of age
- Pulmonary lobectomy in octogenarians with non-small cell lung cancer
- Is laparoscopic colorectal surgery safe also in elderly patients?
- Thyroidectomy in the elderly – its specifics, limitations & contraindications
- Enhanced recovery after colorectal surgery in elderly patients
- Pancreaticoduodenectomy for pancreatic cancer in elderly patients – a single-centre experience
- Perspectives in Surgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Breast cancer in elderly patients over 70 years of age
- Enhanced recovery after colorectal surgery in elderly patients
- Pancreaticoduodenectomy for pancreatic cancer in elderly patients – a single-centre experience
- Pulmonary lobectomy in octogenarians with non-small cell lung cancer
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

