-
Medical journals
- Career
Diagnostics of fatal hydrogen sulfide poisonings
Authors: J. Šidlo; M. Bauer; J. Bauerová; J. Valuch
Authors‘ workplace: Institute of Forensic Medicine, School of Medicine, Comenius University and Healthcare Surveillance Authority, Bratislava, Slovakia
Published in: Soud Lék., 54, 2009, No. 3, p. 37-40
Overview
Introduction:
Cases of fatal hydrogen sulfide poisonings are rarely presented in forensic medicine practice. They mostly occur in occupational settings and occasionally are mass. Due to occupational poisonings caused by gases, hydrogen sulfide is ranked second regarding frequency-dependent incidence. Hydrogen sulfide at high concentrations is undetectable to human senses resulting in increased risk of fatal poisoning. Such poisonings represent a particular group with respect to their objectification by toxicological analysis.Aim:
The aim of this paper is to demonstrate possibilities of laboratory diagnostics of fatal hydrogen sulfide poisonings.Patients and methods:
The paper provides a retrospective overview of 15 cases of fatal hydrogen sulfide poisonings which occurred in the history of the Institute of Forensic Medicine of the School of Medicine, Comenius University in Bratislava. All cases were completely analysed by morphological and toxicological methods. The samples of blood and pulmonary tissue were examined in the toxicological analysis. The method of analysis of alveolar air was developed by Bauer. An experiment for detection of post-mortem production of hydrogen sulfide in the body was performed.Results:
Morphological findings in all autopsied and analysed cases were similar and nonspecific for poisoning diagnosis. A significant change in possibilities of toxicological analysis occurred in 1968. Since then a direct identification of toxic gas through the analysis of alveolar air has been possible.Conclusion:
Taking into consideration summarization and comparison of the analysis results of the cases from archive materials of the authors, it is possible to claim that at the workplace in Bratislava a unique objective method of proving this gaseous poison in biological material used so far has been developed. Some negative or unconvincing results of toxicological analysis again refer to pitfalls of diagnostics of hydrogen sulfide poisonings, which must be complex and based upon an efficient collaboration particularly between a medical examiner and toxicologist-analyst together with other bodies complementing the required spectrum of investigated circumstances and clinical data.Key words:
hydrogen sulfide – fatal poisoning – diagnostics – alveolar air analysisIntroduction
Fatal hydrogen sulfide poisonings belong to rare poisonings not only for their prevalence but also for the manner of their objective evaluation (24). Hydrogen sulfide (H2S) is a flammable, colourless, toxic gas at lower concentrations in air of very foul odour of rotten eggs, at higher concentrations of sickeningly sweet odour. Being slightly heavier than air, it tends to accumulate underneath the ground level, in confined or poorly ventilated spaces, in shafts, holes, etc. Dissolved in water, it forms an unstable solution of hydrosulfuric acid. Its alkali metal salts – sulfides – have clammy effects on the skin and mucosa. Long-term exposure to low concentrations or short-term exposure to high concentrations cause olfactory disorders due to olfactory fatigue and the olfactory nerve paralysis (18). Due to these reasons the odour fails to be an appropriate indicator of hydrogen sulfide presence and cannot warn of dangerous concentrations (7, 8). Hydrogen sulfide occurs in air from both natural and artificial sources. It is produced by decomposition of organic matter particularly of animal origin containing sulfur and proteins, and therefore it occurs in air of sewers, toilets, stable manure piles, septic and sewage tanks, etc. It is also produced within working processes in plants for processing sulfur, in petroleum refineries, in textile industry in production of synthetic fibres, in tanneries, in gasworks, chemical laboratories, etc. (26). It is freely emited from some sulfur mineral springs and volcanoes. The concentration perceived by smell is roughly from 0.3 ppm (7.35 μmol/L) (16). Hydrogen sulfide can enter the body primarily through the airways and a small amount through the intact skin. In the lungs it is well absorbed into blood, where it can readily oxidize to thiosulfides and sulfides. It blocks mitochondrial repiratory string by inhibition of the iron enzyme of cytochromoxidase and lowers the intensity of oxidoreductive processes in tissues causing death resulting from paralysis of tissue and intracellular respiration (31). Other authors see the main toxic effect of hydrogen sulfide in affecting the central nervous system resulting in paralysis of the respiratory and vasomotoric centre (27). Hydrogen sulfide is partially exhaled in an unchanged form and partly is eliminated in the form of metabolites - sulfates, sulfides – in urine (28). The action of hydrogen sulfide on hemoglobin causes its convertion to sulfhemoglobin, which can break down only with difficulty and is not able to transport oxygen. The correlation between outcomes of a toxic action upon humans and the concentration of hydrogen sulfide is shown in Table 1 (29).
1. The correlation between the concentration of hydrogen sulfide in ambient air and its effect on human organism (acc. to Švagr, 1960) 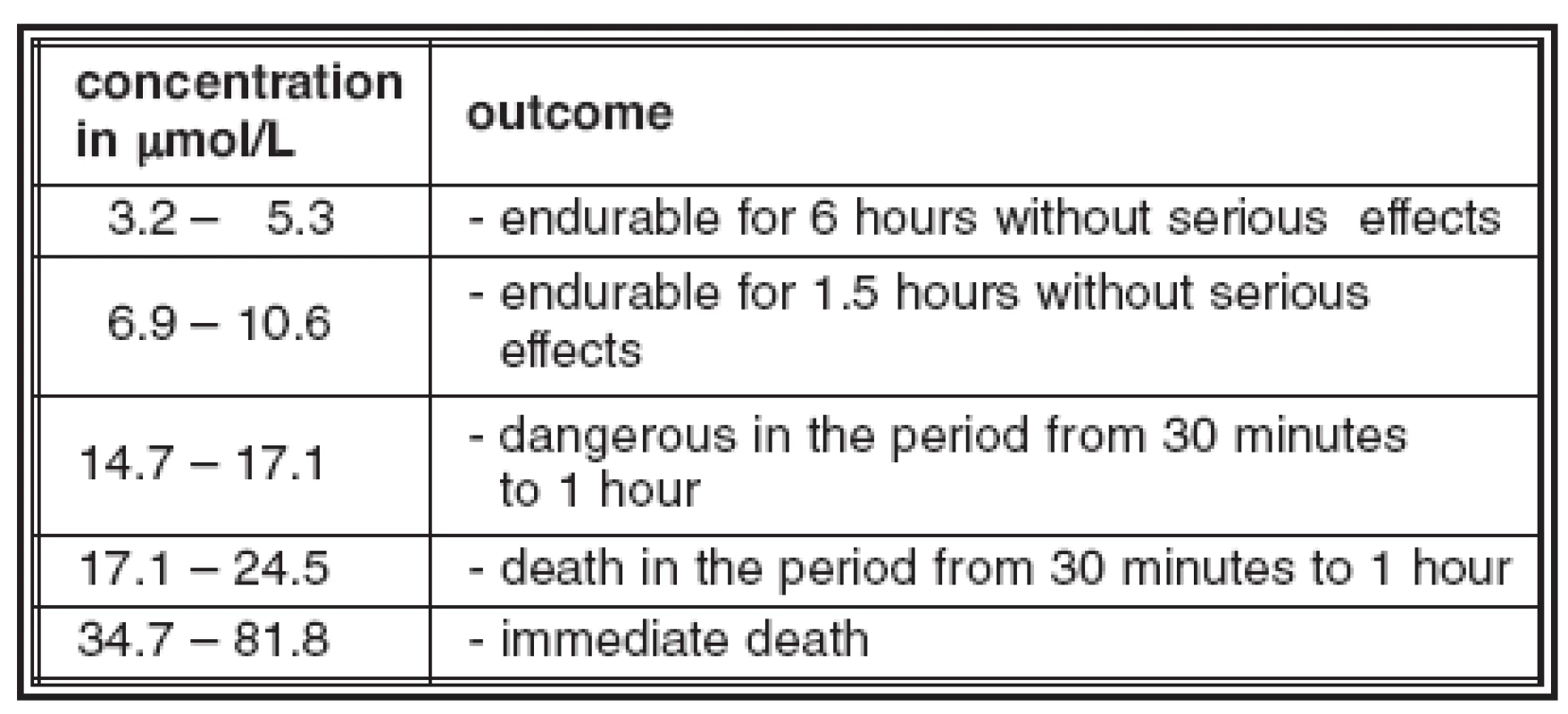
According to Barna (3) the fatal poisoning occurs at a concentration of 61.3 μmol/L; according to Haggart’s data quoted in Legal Medicine, Patology and Toxicology (13) the immediate death occurs at a concentration of 73.6 μmol/L; Adelson (1) refers to a concentration of 57.2 μmol/L as resulting in the immediate death after one or two inhalations. The number of fatal hydrogen sulfide poisonings is relatively low despite its substantial incidence apparently due to the fact that its presence in air is indicated already at low concentrations by its typical and disagreeable odour, which is perceptible according to Lazarev (21) at a concentration ranging from 13.7 to 2.2 μmol/L; according to Wirth et al. (31) it is below 13.7 μmol/L; according to Leith (22) the possibility of olfactory perception is even lower.
Patients and methods
At the Institute of Forensic Medicine of the Faculty of Medicine, Comenius University in Bratislava since 1950 it has been required to give evidence of fatal hydrogen sulfide poisoning in 15 cases during which two types of biological materials were gradually analysed – blood and pulmonary tissue. Sulfhemoglobin was proved in blood by a colorimetric method according to Homolka (15) with the use of a spectral colorimeter SPEKOL (Carl Zeiss Jena, Germany). Following air isolation from the pulmonary tissue initially by a homogenized and later by an evacuating method according to Bauer (4, 5, 6) the presence of hydrogen sulfide was proved with the use of detection tubes KAVALIER (Fig. 1 A - D, 2). A significant precondition of evidence objectivity of hydrogen sulfide in air from the lungs was an experimental examination of postmortem hydrogen sulfide production by putrefaction within which the alveolar air from the lungs was taken from the ten deceased bodies.
Figure 1. Procedure of alveolar air analysis according to Bauer. The weight (A) and volume (B) of the sample of pulmonary tissue is determined. The sample is hermetically enclosed in the vessel from which the air is exhausted by the air pump through the detection tube (C).The resulting underpressure causes destruction of pulmonary alveoli and releasing their content to ambient air allowing identification of toxic gas (D). 
Figure 2. Analysis results of alveolar air. Look at detection tubes after examination of particular lung samples, with a control tube in the middle. Colour alteration determines a positive finding in a detection tube specific for a particular gas. White lines show a level of identified gas concentration. 
Results
Postmortem production of hydrogen sulfide in the experiment was detected ranging from 36 to 96 hours after death. Until 1968 diagnosis of hydrogen sulfide poisoning was established only based on the investigated circumstances, autopsy finding or analysis of ambient air (17). In 1968 hydrogen sulfide poisoning of a worker occurred in a Chemical plant of George Dimitrov (Chemické závody Juraja Dimitrova – CHZJD) in Bratislava, the autopsy was performed 24 hours after death and the evidence of hydrogen sulfide was realised immediately after a separate homogenization of pulmonary tissue in an enclosed space by means of detection tubes; the evidence of hydrogen sulfide above pulmonary tissue was significantly positive. Consecutively acute poisoning occurred in two workers of CHZJD, who had a positive finding of sulfhemoglobin in blood as well as a finding of hydrogen sulfide in air from the lungs. A mass intoxication of three fire fighters occurred in CHZJD in 1973 – during extinguishing the fire above the premises of hydrogen sulfide production. The autopsy and follow-on toxicological analysis were performed 9 hours after death firstly by realizing evidence of sulfhemoglobin colorimetrically according to Homolka (15); secondly isolation of air from the lungs by an evacuation method was performed and its evidence by means of detection tubes – the finding was positive in all cases. In 1973 another hydrogen sulfide poisoning occurred in CHZJD. It was a case of a worker at degassing chambers; the autopsy was performed 5 hours after death with a positive evidence of sulfhemoglobin together with hydrogen sulfide in air from the lungs. In 1978 and 1979 two autopsies were performed in two cases of poisoning after oral ingestion of sulfide. For realizing evidence the pulmonary tissue was not taken, only evidence of sulfhemoglobin was performed. In 1979 during cleaning of sewage a mass on-the-job accident of four workers occurred in a tannery of Bošany. Biological materials were delivered for the analysis as late as 120 hours after death. Since in the experiment the postmortem production of hydrogen sulfide was detected ranging from 36 to 96 hours after death the acquired results could not be objectively evaluated. In 2007 a mass hydrogen sulfide intoxication occurred in Bratislava in a company Agromont in a production hall during sulfurating of colza oil, where two workers died at the scene of accident. The autopsy was performed 22 hours after death. The doctors performing the autopsy benefited from having had foreknowledge as one of them executed the inspection of the bodies at the scene of accident. During the autopsy a toxicological analysis of biological materials was performed concurrently. The presence of sulfhemoglobin in both cases was detected by examining blood samples, in one case methemoglobin was detected as well. In air isolated from the lungs the presence of hydrogen sulfide in one case was detected by means of an evacuation method and follow-on analysis by detection tubes. In another case multiple repeated examination failed to detect the presence of hydrogen sulfide. Additionally it was specified that after the arrival of EMS ambulance on the scene of poisoning there were performed revival attempts yet.
Discussion and conclusions
The pathological and toxicological findings of hydrogen sulfide poisoning vary from case to case. Within postmortem examinations similar morphological findings are determined. Most of authors refer to a partial green discolouration of the skin, pulmonary oedema and a distinct visceral congestion (2, 19, 20, 23). Brain oedema is also determined (11). Christia-Lotter et al. (9) referred to a finding of massive myocardial necrosis in a case of fatal poisoning of a 22-year-old man, a sewer worker. In a case of another sewer worker diffuse necrotic lesions in the lungs were detected (25). From this it follows that morphological findings are not specific for impairment by hydrogen sulfide and they fail to contribute to diagnosing poisoning. Proving fatal hydrogen sulfide poisoning was for long a significant problem also for toxicologists-analysts. A method of proving was discussed by several authors: Gunnina (12) identically with Teisinger (30) submit that determination of hydrogen sulfide in blood has no practical use except for spectroscopic evidence of sulfhemoglobin. Ševčík (28) expressed his opinion, that in proving fatal hydrogen sulfide poisonings only spectroscopic evidence of sulfhemoglobin is appropriate. Wirth et al. (31) indicate that in acute hydrogen sulfide poisining there can be present a characteristic spectrum of sulfhemoglobin, however, largely it is not so. Homolka (14) refers to the level of sulfhemoglobin ranging from 0.5 to 1.0 relat.% as suspicious and the levels above 1.0% as clearly higher. Chu et al. (10) state that sulfured hemoglobin has a specific absorbance peak at 612 μm, and there is a linear relationship with the absorbancity to the concentration of sulfured hemoglobin. Based on the above-mentioned it is possible to mark the poison degree by the spectrophotometry. From the experience acquired by analysing autopsied and toxicologically examined fatal cases of hydrogen sulfide poisoning the following conclusions result: in spite of the fact that hydrogen sulfide poisonings occur rarely, it is necessary to take them into consideration and also in the cases of suspicion of „intoxication by an unknown poison“ it is necessary to take also a sample of pulmonary tissue. In case the investigated circumstances are adequately known and there is a suspicion of poisoning by sulfur compounds, or either after inhaling them or after oral use, it is necessary to perform an autopsy and parallelly a toxicological analysis as soon as possible after assigning death. This fact also resulted from the outcomes of experimental tracking of hydrogen sulfide production in dead bodies post mortem. Establishing diagnosis of fatal hydrogen sulfide poisoning requires an efficient collaboration between police experts, a deceased body examiner, a doctor performing autopsy and toxicologist-analysts.
Acknowledgements
Anna Cibulková, Institute of Foreign Languages, Faculty of Medicine Comenius University Bratislava, Slovakia
Address for correspondence:
Jozef Šidlo, MD., PhD.
Institute of Forensic Medicine, School of Medicine,
Comenius University and Healthcare Surveillance Authority,
Antolská 11,
857 01 Bratislava, Slovakia
tel: ++421259357264, ++421268672349, ++421904819241
fax: ++42163531990
Sources
1. Adelson, L., Sunshine, I.: Fatal hydrogen sulfide intoxication. Arch. Path., 81, 1966, pp. 375–388.
2. Ago, M., Ago, K., Ogata, M.: Two fatalities by hydrogen sulfide poisoning: variation of pathological and toxicological findings. Leg. Med. (Tokyo), 10, 2008, pp. 148–152.
3. Barna, K.: Introduction to medical chemistry. Martin, Osveta, 1975, pp. 943. (in Slovak)
4. Bauer, M.: Isolation of air from the lungs of the deceased for the purpose of toxicological analysis. Z. Rechtsmedizin, 73, 1973, pp. 115–118. (in German)
5. Bauer, M.: Method and device for isolating residual pulmonary air from cadaverous material for separation of toxic or other components. Authorial certificate. ČSSR 171795. PV 8113–72, 1972. (in Slovak)
6. Bauer, M.: Method of isolating residual pulmonary air from cadaverous material and separation of particularly toxic components of isolated air and device for performing of this method. Authorial certificate. ČSSR 172517. PV 1295-73, 1973. (in Slovak)
7. Buchancová, J. et al.: Occupational medicine and toxicology. Martin, Osveta, 2003, pp. 1133. (in Slovak)
8. Chaturvedi, A.K., Smith, D.R., Canfield, D.V.: A fatality caused by accidental production of hydrogen sulfide. Forensic Sci. Int., 123, 2001, pp. 211–217.
9. Christia-Lotter, A., Bartoli, C., Piercecchi-Marti, M.D., Demory, D., Pelissier-Alicot, A.L., Sanvoisin, A., Leonetti, G.: Fatal occupational inhalation of hydrogen sulfide. Forensic Sci. Int., 169, 2007, pp. 206–209.
10. Chu, J.X., Man, Q., Bao, C.S.: Determination of the hemoglobin in poisoned blood by spectrophotometery. Fa i Hsueh Tsa Chih Journal of Forensic Medicine. 19, 2003, pp. 212–214. (in Chinese)
11. Fialová, J., Slaný, J., Nakládal, Z., Nakládalová, M., Kosatík, A., Bartoušek, J.: Mass hydrogen sulfide poisoning. Pracov. Lék., 49, 1997, pp. 177–181. (in Czech)
12. Gunnina, A.J., Tichonravov, V.A.: Farmakol. i Toxikol. 16, 1953, p. 46
13. Haggart, A.: Legal Medicine, Pathology and Toxicology. New York, Appleton-Century-Crofts, 1954, pp. 99.
14. Homolka, J.: Clinical biochemistry. Praha, Avicenum, 1971, pp. 508. (in Czech)
15. Homolka, J.: Clinical biochemical investigating methods. Praha, SZdN, 1969, pp. 438. (in Czech)
16. Hydrogen sulfide, Available on: <http://www.ruvzbj.sk/> (in Slovak)
17. Kimura, K., Hasegava, M., Matsubara, K., Maseda, C., Kagawa, M., Takahashi, S., Tanabe, K.: A fatal disaster case based on exposure to hydrogen sulfide – an estimation of the hydrogen sulfide concentration at the scene. Forensic Sci. Int., 66, 1994, pp. 111–116.
18. Knight, L.D., Presnell, S.E.: Death by sewer gas: case report of a double fatality and review of the literature. Am. J. Forensic. Med. Pathol, 26, 2005, pp. 181–185.
19. Kohout, J., Slabý, O., Bejčková, H., Šamanová, M.: Mass hydrogen sulfide poisoning in a paper-mill. Pracov. Lék., 40, 1988, pp. 249–251. (in Czech)
20. Kučera, R., Kohout, J., Slabý, O., Bejčková, H., Šamanová, M.: Complex therapy of severe hydrogen sulfide poisoning. Prakt. Lék., 68, 1988, pp. 765-766. (in Czech)
21. Lazarev, N.V.: Chemical poisons in industry. Praha, SZdN, 1959, I., pp. 724. (in Czech)
22. Leithe, W.: Analysis of air and its pollution in ambient air and at workplace. Stuttgart, Wissenschaftliche Verlagegesellschaft MBH, 1974, pp. 303. (in German)
23. Marhold, J.: Review of industrial toxicology. Anorganic substances. Praha, Avicenum, 1980, pp. 522. (in Czech)
24. Osina, O., Buchancová, J., Berešík, M., Kozák, P., Onderčanin, M., Bodáková, D.: Occupational acute hydrogen sulfide poisoning. České pracovní lékařstvi, 2007, pp. 64–69. (in Slovak)
25. Querellou, E., Jaffrelot, M., Savary, D., Savry, C., Perfus, J.P.: Fatal accidental hydrogen sulfide poisoning. Annales Francaises d’Anesthésie et de Réanimation, 24, 2005, pp. 1302–1304. (in French)
26. Reichl F. X.: Pocket atlas of toxicology. Hamburg, Nikol Verlag, 2nd ed., 2008, pp. 349. (in German)
27. Summary of hydrogen sulfide, Geneva, World Health Organization, 1981 (Environmental Health Criteria No. 19), pp. 48.
28. Ševčík, M.: Practical toxicology. Praha, SZdN, 1965, pp. 313. (in Czech)
29. Švagr, E.: Basis of chemical toxicology. Praha, SZdN, 1960, pp. 192. (in Czech)
30. Teisinger, J., Škramovský, S., Srbová, S.: Chemical methods for investigating of biological material in industrial toxicology. Praha, SZdN, 1956, pp. 127. (in Czech)
31. Wirth, W., Hecht, G., Gloxhuber, Ch.: Handbook of toxicology. Stutgart, Georg Thieme Verlag, 1971, pp. 469. (in German)
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inForensic Medicine

2009 Issue 3
Most read in this issue- Diagnostics of fatal hydrogen sulfide poisonings
- Problems on Czech Anatomical Nomenclature in Forensic Medicine
- Death of Pedestrians after Trafic Accidents with Motor Cars
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career




