-
Medical journals
- Career
From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
Authors: Oldřich Farsa; Pavol Brka
Authors‘ workplace: Department of Chemical Drugs, Faculty of Pharmacy University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic
Published in: Čes. slov. Farm., 2015; 64, 287-288
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Barbiturates are old-fashioned and mainly obsolete sedatives, hypnotics and antiepileptics. Only phenobarbital (Phenaemal®, Phenaemaletten®) remains approved as a reserve medicine for epilepsy. Symmetrical barbiturates such as allobarbital (5,5-diallylbarbituric acid) or 5,5-dipropylbarbituric acid were mainly used in irrational combinations with weak analgesics and are completely obsolete. On the contrary, valproic acid and its salts are modern and popular therapeutics which are used not only as antiepileptics. Symmetrical barbiturates were being synthetized in our department practical courses during the 1990’s. We have thus hardly utilizable reserves of these compounds. Some of them are classified as psychotropic compounds in accordance with the Addictive Compounds Act1) and the Government Regulation Concerning the Lists of Addictive Compounds2). It is desirable to get rid of such reserves in accordance with the law. That is why we have proposed to use our old department reserves of 5,5-dipropylbarbituric acid as the starting material for a new experiment in our Medicinal Chemistry practical courses in which valproic acid will be prepared.
Experimental methods
Experiments involved optimization of two synthetic steps: hydrolysis of 5,5-dipropylbarbituric acid to 2,2-dipropylmalonic acid and decarboxylation of the latter to 2-propylpentanoic, e.g. valproic acid. The first synthetic step had to be optimized because the composition of products of the alkaline hydrolysis depends on the reaction conditions. Such hydrolysis can result in 2,2-dipropylmalonuric acid or in mono - or diamide of 2,2-dipropylmalonic acid except or instead of the desirable 2,2-dipropylmalonic acid3, 4) (Fig. 1). These problems were solved by usage of enough excess of sodium hydroxide, its optimal concentration and the satisfactory reaction time. The second synthetic step is a thermal decomposition – decarboxylation of 2,2-dipropylmalonic acid. It has been optimized for performance in a microwave reactor where it, under catalysis with pyridine, takes only a few minutes5). The product is then isolated by dissolving of the reaction mixture in water, acidification and extraction of the product into diethyl ether. In need it can be distilled under reduced pressure, typically using a ball-to-ball distillation apparatus. We had also found conditions for the conventional version of this reaction which requires copper(I) oxide catalysis6). This version takes about 30 minutes (Fig. 2).
1. Hydrolysis and other reactions of 5,5-dipropylbarbituric acid in an alkaline hydroxide solution 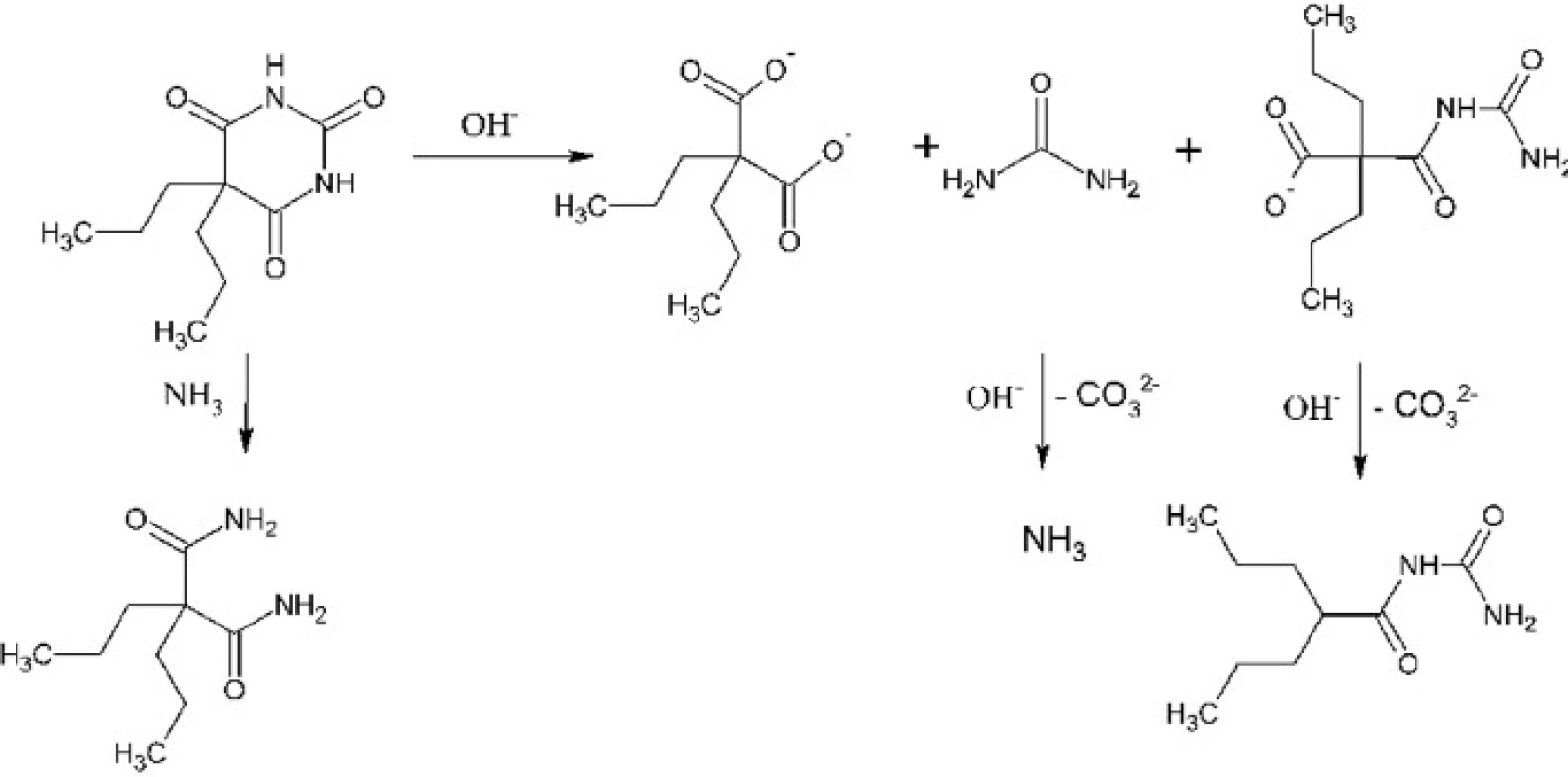
2. The overall scheme of synthesis of valproic acid from 5,5-dipropylbarbituric acid (or from allobarbital) 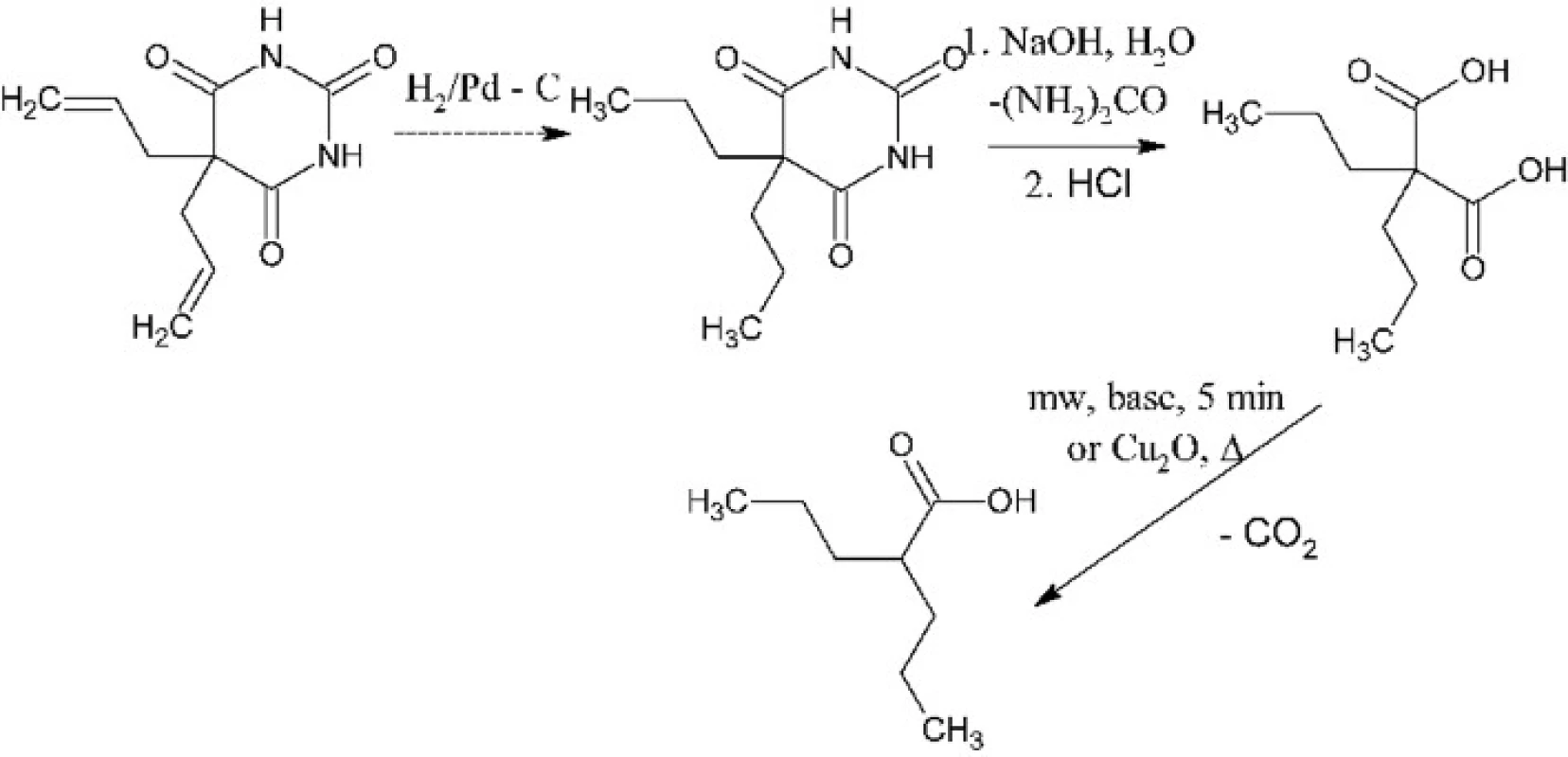
Results and discussion
The two-stage synthetic procedure for synthesis of valproic acid has been developed. The first stage is alkaline hydrolysis of 5,5-dipropylbarbituric acid to 2,2-dipropylmalonic acid in a larger amount. This step will be performed by a laboratory technician to avoid the contact of students with the barbiturate. The second stage is the thermal decarboxylation of 2,2-dipropylmalonic acid to valproic acid. This step will be performed by students either in a microwave reactor or by a conventional heating. It must be noted that one-pot syntheses of α-substituted alkanoic acids directly from a barbiturate have also been reported7) but they take at least 24 hours which is inapplicable in practical classes. When the reserve of 5,5-dipropylbarbituric acid will be consumed, we will hydrogenate our reserve allobarbital to get it (Fig. 2). After consumption of allobarbital, the first stage will be replaced with a conventional synthesis of 2,2-dipropylmalonic acid from diethyl malonate.
Conclusions
A simple two-step synthetic procedure which enables the synthesis of valproic acid from 5,5-dipropylbarbituric acid suitable for practical courses has been developed. The slower first step, hydrolysis of 5,5-dipropylbarbituric acid to 2,2-dipropylmalonic acid, is intended to be performed by a laboratory technician to prepare a reserve of this intermediate while the second step, decarboxylation of 2,2-dipropylmalonic acid to 2-propylpentanoic (valproic) acid will be made by students.
Conflicts of interest: none.
doc. PharmDr. Oldřich Farsa, Ph.D.
Department of Chemical Drugs, Faculty of Pharmacy
University of Veterinary and Pharmaceutical Sciences
Palackého 1/3, 612 42 Brno, Czech Republic
e-mail: farsao@vfu.cz
Sources
1. Addictive Compounds Act No. 167/1998 Coll.
2. Government Regulation Concerning the lists of Addictive Compounds No. 463/2013 Coll.
3. Aspelund H., Skoglund L. The stability of the sodium salts of some soporifics of the barbituric acid series in solution. Acta Acad. Aboensis Math. et Phys. 1937; 10, 14–36.
4. Mokrosz J., Bojarski J. Hydrolysis of barbituric acid derivatives. Part IV. Hydrolysis of derivatives without a shielding steric effect. Polish Journal of Chemistry 1982; 56, 491–500.
5. Helavi V. B., Solabannavar S. B., Desai U. V., Mane R. B. Microwave assisted hydrolysis of Meldrum’s acid derivatives and decarboxylation of derived malonic acids. J. Chem. Research (S), 2003; 2003, 174–175.
6. Toussaint O., Capdevielle P., Maumy M. The Copper(I)-Catalyzed Decarboxylation of Malonic Acids; A New Mild and Quantitative Method. Synthesis 1986; 1986. 1029–1031.
7. Barton H., Mokrosz J., Bojarski J., Klimczak M. Photochemical degradation of barbituric acid derivatives. Part 1. Products of photolysis and hydrolysis of pentobarbital. Pharmazie 1980; 35, 155–158.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2015 Issue 6-
All articles in this issue
- Antibacterial activity of natural compounds – essential oils
- Cholinergic system of the heart
- Body surface area and body weight of Czech adult cancer population
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Antibacterial activity of natural compounds – essential oils
- Body surface area and body weight of Czech adult cancer population
- Cholinergic system of the heart
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

