-
Medical journals
- Career
Effects of Mg-ATP on the flavonoid production in the Scutellaria baicalensis Georgii suspension cultures
Authors: Z. Kršková; J. Martin; J. Péč; J. Dušek
Authors‘ workplace: Charles University in Prague, Faculty of Pharmacy, Department of Pharmacognosy
Published in: Čes. slov. Farm., 2008; 57, 111-114
Category: Original Articles
Overview
This work focused on the cultivation of S. baicalensis Georgii in vitro cultures and on the possibilities of increasing the production of secondary metabolites in these cultures. The aim of the study was to determine whether the baicalin transport through vacuolar membrane is dependent on the presence of Mg-ATP. Our results showed that Mg-ATP had a significant effect on the ratio of baicalin and baicalein content and on the transport speed of these flavonoids. Therefore, the transport mechanism for baicalin are probably some of the MRP proteins which are the subfamily of the ABC transporters.
Key words:
Scutellaria – baicalin – baicalein – Mg-ATP – ABC transportersIntroduction
Vegetable drugs are usually obtained either by their direct collection in nature or by farming them in fields and gardens. These sources, however, have a whole line of disadvantages, for instance, the dependency on climatic and geographic conditions or the possibility of being attacked by pests. These factors often lead to the fluctuation in the quality and quantity of the vegetable drug.
The answers to some of these problems might rest in applying biotechnological methods that use cultures of vegetable cells, vegetable tissues, and vegetable organs cultured in vitro. These so-called explant cultures can be obtained from any plant and they offer the advantage of fully controllable and fully reproducible conditions. Their other advantages include, above all, the non-dependency on climate, weather and soil, the extracted products are pesticide free and insecticide free, are homogenous and sterile, and can be genetically modified more easily.
In case of the explant cultures of medical plants, the primary objective is to increase the production of therapeutically important substances. Elicitation, for instance, can help us to do so. This method consists in the fact that the elicitor (of biotic or abiotic origin) causes a stress situation in a cell and the cell can respond with an increased production of secondary metabolites.
Scutellaria baicalensis Georgii, Lamiaceae, the native to Asia, is a medical plant that is widely used in traditional Chinese medicine. It is used as antipyretic, antihypertensive, sedative, and to treat inflammations, allergies and insomnia 1, 2). It also serves to eliminate subjective symptoms such as headaches and pains in the heart area. Concerning its antioxidant and antiviral effects, it is also a potential cytostatic agent 3).
The main content substances of this plant are flavonoids of which the most important one is baicalin and its aglycone baicalein.
The storage of baicalin in the cells of Scutellaria baicalensis Georgii, as well as the storage of other flavonoid glycosides, takes place in vacuoles. In the event of a need, for instance when a plant cell is exposed to oxidative stress, baicalin is transported into cytosol and, during this process, it loses its sugar component, changes into baicalein, and transfers through tonoplast into cytosol. In cytosol, baicalein reacts with the oxygen radical and with the aid of peroxidase it is then transferred into 6,7-dehydrobaicalein 4).
Most of the secondary metabolism substances are transported in glycosylated state through tonoplast utilizing the proton-substrate antiport. These secondary metabolites, able to carry the minus charge (for example glucuronides), are caught by the direct energetic utilization ATP. The proteins that provide for this transport are classified as ABC transporters, or more precisely, as the subfamily of the MRP proteins. The mechanism mentioned above could be used to transport the flavonoid glucuronide baicalin 5).
This work is focused on the observation of effects of the elicitors of the methylene blue and of the hydrogen peroxide with addition of an Mg-ATP molecule on the production of baicalin flavonoid and on the production of its baicalein aglycone in the Scutellaria baicalensis Georgii suspension culture.
EXPERIMENTAL PART
Chemicals
- Methanol for HPLC: Fluka, Buchs;
- baicalin p.a., baicalein p.a., Mg-ATP p.a.: Sigma Aldrich Chemie, Steinheim;
- α-naphtyl acetic acid p.a., myoinositol p.a..: Sigma, St. Luis;
- thiaminium chloride p.a., pyridixinium chloride p.a.: Koch-Light Laboratories Ltd., Colnbrook Hampshire;
- enzymatic hydrolyzate of casein: Imuna, Sarisske Michalany;
- hydrogen dioxide: Penta, Chrudim;
- potassium dihydrogen phosphate p.a., nitrate of potash p.a., nitrate of ammonium p.a., glycin p.a., sodium hydrogen phosphate p.a., chloride of cobalt p.a., chloride of calcium p.a., iodide of potassium p.a., boracic acid p.a., o-phosphoric acid p.a., nicotinic acid p.a., methanol p.a., methylene blue for micro, sodium molybdate p.a., light petroleum p.a., saccharose p.a., sulphate of magnesium p.a., sulphate of manganese p.a., sulphate of copper p.a., sulphate of zinc p.a., sulphate of iron p.a.: Lachema, Brno
Biological material
The suspension culture was derived from the root parts of the Scutellaria baicalensis Georgii plant in the 49th and 50th passage of the experimental work.
Cultivation of the explant culture
The suspension cultures were derived from callus cultures by mechanical loosening of the calluses. The cultivation took place in 250 ml flat bottom flasks positioned on rotary shaker (set at 120 rpm-1). The Murashige and Skoog nutrient medium was used with the addition of the growth stimulator of α naphtyl acetic acid in the concentration of 10 mg.l-1. The flat bottom flasks contained 50 ml of the media and the cultivation period was 12–14 days. The cultivation took place during the 16-hour light period at the temperature of 25 °C. The passage of the suspension cultures was done with sterile pipettes.
Elicitation
Observing the effects of Mg-ATP and methylene blue
We monitored the production of secondary metabolites in the suspension cultures after the elicitation with oxygen radical that was being created directly in the medium by the methylene blue.
The methylene blue was added to the first group of suspension cultures at the end of their cultivation period. This elicitor was always added in the amounts of 1 ml that were taken from sterile, reserve solutions so that the resulting concentration in the flasks would amount to 100 mg.l-1. The other group of cultures received the methylene blue plus 1 ml of the Mg-ATP solution in the same manner (the resulting concentration in the flasks amounted to 3 mmol.l-1; taken from the work of Walczak et al 6) ). The control cultures were supplemented with 1 ml of sterile water. The subsequent cultivation took place during a 16-hour light period at the temperature of 25 °C. The culture samples were taken after 180, 300, and 1440 minutes. After drying and extracting them, we used the HPLC method to determine the contents of baicalein and baicalin.
Observing the effects of Mg-ATP and hydrogen peroxide
At the end of their cultivation period, hydrogen peroxide was applied to the suspension cultures as the elicitor; always in 1 ml amounts taken from sterile, reserve solution until the resulting concentration in the flasks amounted to 13.6 mg.l-1 (corresponds to 4 μmol.g-1, taken from the work of Morimoto et al 4)). In addition to H2O2, the other cultures were also supplemented with 1 ml of the Mg-ATP reserve solution (the resulting concentration in the flask was 3 mmol.l-1). The control cultures were supplemented with 1 ml of sterile water. The subsequent cultivation took place during a 16-hour light period at 25 °C. The samples were taken after 30, 60, 180, and 300 minutes. The HPLC method was used to determine the contents of baicalein and baicalin.
Determining the contents of baicalin and baicalein flavonoids
The suspension cultures were first filtered and washed in water. Then they were left to dry on filter paper for 24 hours and, subsequently, their drying was completed in a dryer set at 50 °C. The dried material was pulverized in a mortar and extracted: the sample weighting 0.2000 g was extracted twice with 10 ml of 80% methanol on water bath under reflux cooler for the duration of 30 minutes. After the extraction, the volume was replenished to 20 ml with 80% methanol. Chlorophyll and lipids were removed during multiple shaking in light petroleum. The samples were then filtered through teflon micro filter (0.45 μm) and were ready for the HPLC analysis
HPLC analysis
The HPLC analysis was carried out utilizing the Jasco assembly of instruments (PU-2089 pump, MD-2015 detector, AS-2055 autosampler). The assembly was equipped with fore-column filter and LiChrospher RP-18 column, 250 × 4 mm with protective fore-column.
The injected volume was 20 μl. The mobile phase composition took place in the linear gradient from 50% methanol with the content of 0.15% phosphoric acid (pH = 2.9) at time t = 0 to 75% methanol with the content of 0.15% phosphoric acid at time t = 15 minutes at the mobile phase’s constant flow rate of 1.2 ml.min-1.
The detection was carried out with the DAD detector in the wave length range of 190–450 nm. The content of the monitored flavonoids was calculated from the peaks at the wave length of 277 nm, at which both flavonoids have their absorption maximums. The retention time of baicalin was approximately 6 minutes and 33 seconds and in case of baicalein it was approximately 11 minutes and 40 seconds. The contents of both substances were quantified by using mathematical method of normalization and by comparing with the calibration curve drawn by the external standard of the same substance.
RESULTS
The results of the chemical analysis of the contents of the flavonoids of baicalin and baicalein in explant cultures are listed in tables. All the listed results are the averages calculated from the results of three independent determination processes.
Discussion
The objective of this experimental work was to determine whether the transport of baicalin through tonoplast is subject to the presence of Mg-ATP, and whether Mg-ATP could be used as the elicitor to increase the flavonoid contents in in vitro cultures.
The Scutellaria baicalensis Georgii suspension culture was tested for the effects of Mg-ATP in the presence of substances that produce oxygen radicals. The selection of the lengths of time intervals and of the concentrations of substance was based on previous experiments with elicitors – the methylene blue and the hydrogen peroxide 7). The results are listed in tables 1 and 2. The two substances mentioned above were used as the producers of the oxygen radical.
1. Effect of the methylene blue (100 mg.l-1) and Mg-ATP (1,594 g.l-1) on the Scutellaria baicalensis Georgii suspension culture 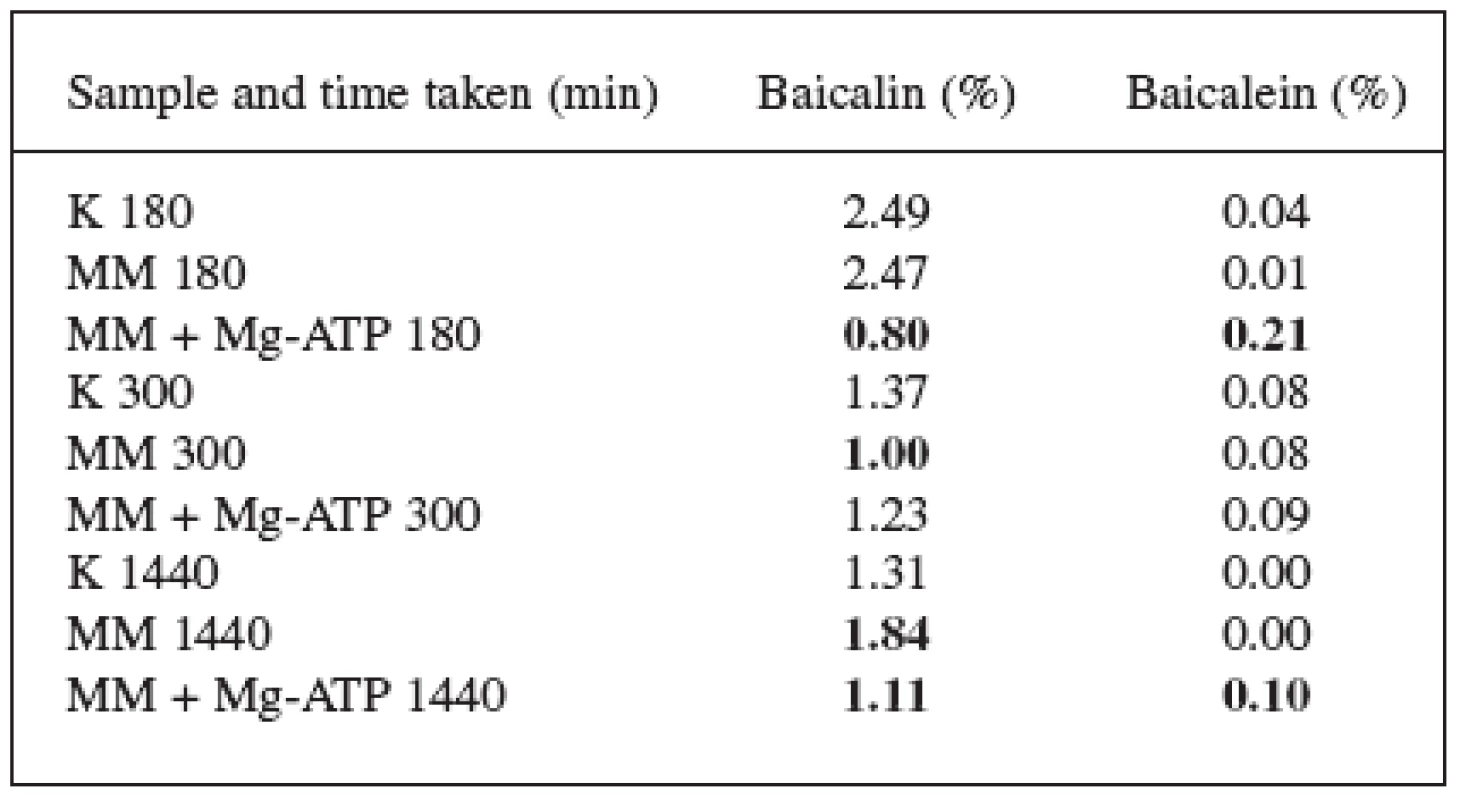
K – check, MM – methylene blue, Mg-ATP – adenosine triphosphate magnesium salt statistically important values are in bold 2. Effect of H2O2 (13,6 mg.l-1) and Mg-ATP (1,594 g.l-1) on the Scutellaria baicalensis Georgii suspension culture 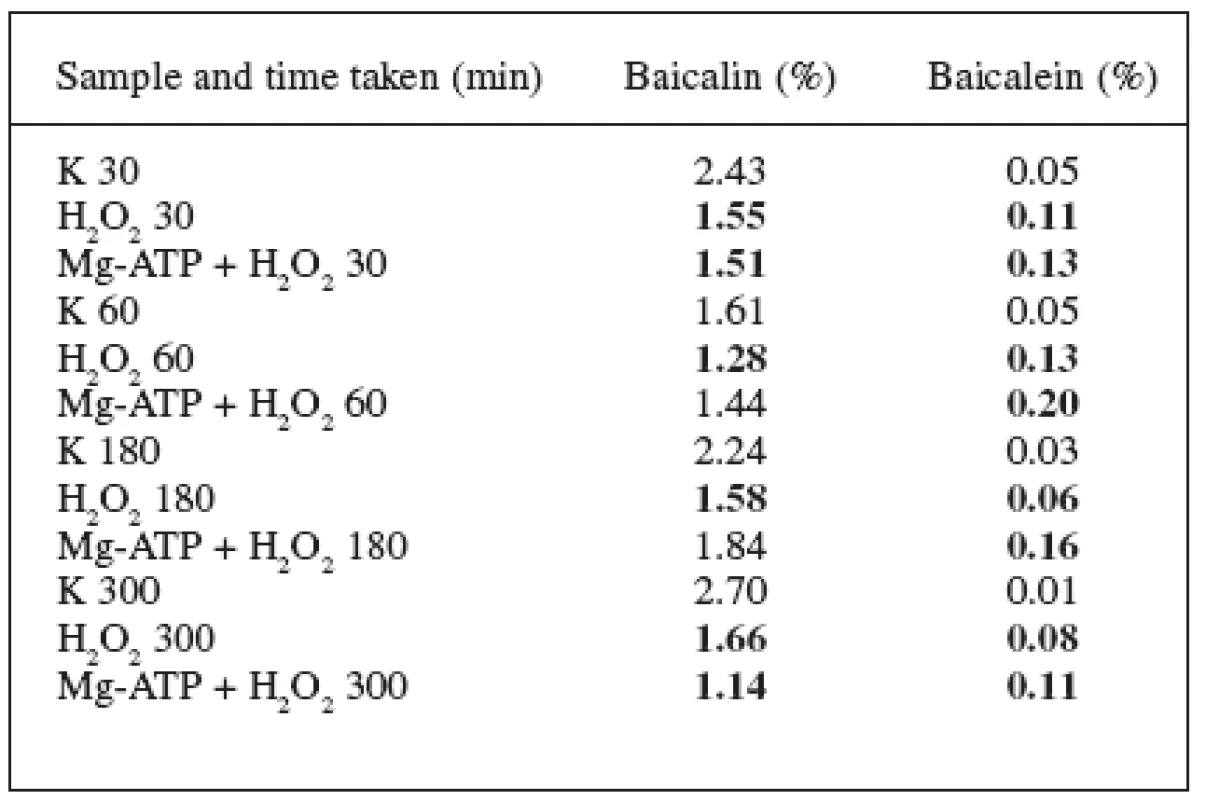
K – check, H2O2 – hydrogen peroxide, Mg-ATP – adenosine triphosphate magnesium salt, statistically important values are in bold Methylene blue is a water-soluble dye that is used in dying of microscopic specimens. Since it does not penetrate the cell walls of live cells, it cannot elicitate with the direct intervention in the secondary metabolism of the cell, but it can have an indirect effect as the generator of active forms of oxygen 8). In the presence of light and molecular oxygen, this substance produces the oxygen radical and, indirectly, also other active forms of oxygen – peroxides and superoxides. These active forms of oxygen threaten cells, among other, by damaging the DNA 9, 10).
The hydrogen peroxide effect principle is similar to the one of the methylene blue – it is the active form of oxygen. But after adding this substance, there is an immense forced increase in the amount of peroxide in the medium which the detoxication systems of the cell are able to decrease with time (in case of the methylene blue, the continual production of active forms of oxygen is in negligible amounts).
The molecule of adenosine triphosphate (ATP) serves as the source of energy for a number of cell reactions. In this experimental work, we used its magnesium salt. Mg-ATP, when added to plant cells, should speed up the transport of substances through tonoplast and our work tries to confirm this fact.
The effect of the methylene blue alone was presented in the thesis by Jan Martin 7). In this quoted work, the methylene blue caused an increase in the content of baicalin after 8 and 24 hours and proved to be a suitable elicitor for S. baicalensis cultures.
These results were confirmed and updated with the 180 and 300 minutes (3 and 5 hours) figures. The content of baicalin in these time periods decreased but, after 1440 minutes (24 hours), it again increased. The results of both pieces of work indicate that the baicalin biosynthesis starts between 300 and 1440 minutes after the elicitation with the methylene blue.
180 minutes after adding Mg-ATP and the methylene blue, there was a radical decrease in the content of baicalin and an increase in the content of baicalein. The content of baicalin decreased from 2.49% (check) to 0.80%; the content of baicalein increased from 0.04% to 0.21%. The loss of baicalin was not that substantial after 300 and 1440 minutes which can be explained by the starting synthesis of baicalin and by replenishing its content in the vacuoles. The content of baicalin at the 1440-minute time mark dropped from 1.31% to 1.11%, the content of baicalein increased from 0.00% to 0.10%.
Considering the different results we arrived to when using methylene blue alone and methylene blue in combination with Mg-ATP, we can state that Mg-ATP significantly affects the contents of both flavonoids and has direct effect on the transport of baicalin flavonoid across the vacuolar membrane and thus also on the associated hydrolysis to baicalein and glucuronic acid.
The effect of hydrogen peroxide in the combination with Mg-ATP did not significantly differ when compared with the effect of the hydrogen peroxide alone – the content of baicalin slightly decreased and the content of baicalein slightly increased. The most significant change was recorded at the 300-minute time when the content of baicalin, in comparison with the check, decreased from 2.70% to 1.14% and the content of baicalein increased from 0.01% to 0.11%.
The effect of the Mg-ATP molecule on the form of flavonoids was thus observed in these experiments as well. The molecule caused a decrease in the content of baicalin due to its transformation to baicalein by cleaving away the glucuronic acid on the transporter. The increase in the baicalein content was not very significant, apparently because of the fast oxidation to 6.7 dehydrobaicalein.
The results indicate that Mg-ATP positively affected the transport speed of baicalin between the vacuole and cytosol. The baicalin transport mechanism is most likely the MRP proteins that are the subgroup of the ABC transporters.
This work was sponsored under the MSM 0021620800 research project.
Received 26 February 2008
Accepted 28 March 2008
Corresponding author:
PharmDr. Jan Martin, Ph.D.
Farmaceutická fakulta v Hradci Králové, Katedra farmakognozie
Heyrovského 1203, 500 05 Hradec Králové
e-mail: martin@faf.cuni.cz
Sources
1. Hoffman, D.: The Herbal Handbook: A User’s Guide to Medical Herbalism, Rochester, Healing Arts Press, 1988, s.77.
2. Cuellar, M. J. et al.: Fitoterapia, 2001; 72, 221.
3. Konoshima, T. et al.: Chem. Pharm. Bull., 1992; 40, 531.
4. Morimoto, S. et al.: J. Biol. Chem., 1998; 273, 12606-12611.
5. Klein, M. et al.: Phytochemistry, 2001; 56, 153-159.
6. Walczak, H. A., Dean, J. V.: Phytochemistry, 2000; 53, 441-446.
7. Martin, J.: Dizertační práce, Hradec Králové, Univerzita Karlova, Farmaceutická fakulta, 2006 - Martin, J.: Thesis, Hradec Kralove, Charles University, Pharmaceutical Faculty, 2006
8. Sakaki, H. et al.: J. Biosci. Bioeng., 2002; 93, 338.
9. Epe, B., Pflaum, M., Boiteux, S.: Mutat. Res-Gen. Tox. En., 1993; 299, 135.
10. Tuite, E. M., Kelly, J. M.: J. Photoch. Photobiol. B, 1993; 21, 103.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2008 Issue 3-
All articles in this issue
- Synthesis of 2-{3-[4-(4-fluorophenyl)-1-piperazinyl]-2-hydroxy-propoxy}-phenylcarbamic acid alkylesters and in vitro evaluation of their ß-antiadrenergic and vasodilatative activities
- Studies on local anesthetics Part 183: Micellization and thermodynamic parameters of heptacainium chloride in the solution of potassium bromide
- Medicinal preparations in Mattioli’s Herbarium of 1596
- On the content of the national part of the Czech Pharmacopoeia from the aspect of the formulation of medicinal preparations in pharmacies
- Biotic elicitation of the L. suspension culture
- Effects of Mg-ATP on the flavonoid production in the Scutellaria baicalensis Georgii suspension cultures
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Medicinal preparations in Mattioli’s Herbarium of 1596
- On the content of the national part of the Czech Pharmacopoeia from the aspect of the formulation of medicinal preparations in pharmacies
- Studies on local anesthetics Part 183: Micellization and thermodynamic parameters of heptacainium chloride in the solution of potassium bromide
- Synthesis of 2-{3-[4-(4-fluorophenyl)-1-piperazinyl]-2-hydroxy-propoxy}-phenylcarbamic acid alkylesters and in vitro evaluation of their ß-antiadrenergic and vasodilatative activities
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

