-
Medical journals
- Career
Central Serous Chorioretinopathy. A Review
Authors: K. Myslík Manethová 1,2
Authors‘ workplace: Oční oddělení, Fakultní Thomayerova nemocnice, Praha 1; Fakultní nemocnice Královské Vinohrady a 3. lékařská fakulta Univerzity Karlovy, Praha 2
Published in: Čes. a slov. Oftal., 3, 2023, No. Ahead of Print, p. 1001-1013
Category: Review
doi: https://doi.org/10.31348/2023/27Overview
Central serous chorioretinopathy (CSC) is a disease characterized by serous detachment of the neuroretina, especially in the posterior pole of the eye. It is often accompanied by serous detachment of the retinal pigment epithelium (RPE) and associated with the leakage of fluid into the subretinal space through the defective RPE. CSC most often affects men of working age. The exact pathophysiology of the disease is not completely known. Based on indocyanine green angiography (ICG), which revealed increased permeability of choroidal vessels, and optical coherence tomography (OCT) showing increased choroidal thickness, choroidal vasculopathy is assumed to be the primary cause of CSC. In most cases, CSC has a good prognosis with spontaneous resorption of the subretinal fluid (SRF) and improvement of visual functions. However, in a small percentage of patients the disease progresses to a chronic or recurrent course, and can lead to irreversible functional and anatomical changes of the retina with a final clinical picture of diffuse retinal pigment epitheliopathy (DRPE). The optimal treatment approach for patients with CSC remains controversial. In recent decades, myriad therapeutic approaches have been used in the treatment of chronic forms of CSC (cCSC); these included for example laser photocoagulation, pharmaceutical treatment, standard photodynamic therapy (PDT) or anti-VEGF. In recent years a less destructive method, specifically PDT in reduced dose regimens, either with a reduced dose of verteporfin or the laser beam energy used, has been preferred in the treatment of cCSC. Comparable efficacy and safety has been demonstrated using reduced-dose or reduced-fluence PDT regimens in patients with cCSC, with an improvement in best-corrected visual acuity and reduction of SRF.
Keywords:
Retinal degeneration – photodynamic therapy – central serous chorioretinopathy – choroidal neovascularization – multimodal imaging
Central serous chorioretinopathy (CSC) is a pathology characterized by serous detachment of the neuroretina, especially in the region of the posterior pole of the eye [1]. It is frequently accompanied with serous detachment of the retinal pigment epithelium (RPE) and associated with the leakage of fluid into the subretinal space through the defective RPE. CSC most often affects men of working age [2]. The precise physiopathology of the disease is not entirely known. On the basis of angiographic examination with indocyanine green (ICG), which detected increased permeability of the choroidal vessels [3], and optical coherence tomography (OCT) showing increased choroidal thickness [4], choroidal vasculopathy is considered to be the primary cause of CSC.
In the majority of cases, CSC has a good prognosis, with spontaneous resorption of the subretinal fluid (SRF) and improvement of visual functions. However, in a small percentage of patients the pathology progresses to a chronic or recurrent course, and may lead to irreversible anatomical changes of the retina, with a final clinical picture of diffuse retinal pigment epitheliopathy (DRPE) [3].
The optimal therapeutic approach to patients with CSC remains controversial. In recent decades countless therapeutic approaches have been used in the treatment of chronic forms of CSC (cCSC); these have included for example laser photocoagulation [5], pharmaceutical treatment [6,7], standard photodynamic therapy (PDT) [8] or agents acting against vascular endothelial growth factor (anti-VEGF) [9]. In recent years, a less destructive method has been preferred in the treatment of cCSC, specifically PDT in reduced dose regimens (rPDT), whether this concerns a reduced dose of verteporfin or reduced used energy of the laser beam [10–12]. Alkin, Chan and Uetani have demonstrated comparable efficacy and safety in the use of reduced-dose or reduced-fluence PDT in patients with cCSC, in whom an improvement of best corrected visual acuity (BCVA) and a reduction of SRF was achieved, without any complications being recorded in the observed cohort [13–15].
No standards exist in the Czech Republic for the treatment of this clinical unit. Because the disease typically affects younger patients of working age with high demands for quality of vision, correct timing and accurate designation of treatment remain an acute challenge, especially in the case of chronic form of CSC. Whereas treatment previously relied exclusively on laser treatment of the retina [16], in recent years more conservative methods have been favored particularly in the treatment of chronic forms of CSC, such as PDT or intravitreal application of antiangiogenic substances [17,18].
HISTORY
Historically, a pathology of the macula with recurrent serous retinal detachment was first described in 1866 by von Graef, termed recurrent central retinitis [19]. Almost one hundred years later in 1955, Bennet used the term central serous retinopathy, since he believed that the fluid originated from leaking retinal capillaries, and not upon the background of inflammation or angiospasm [20]. In the 1960s, thanks to the introduction of fluorescence angioscopy into clinical practice, Maumenee determined that the fluid was penetrating beneath the retina through the damaged RPE [21]. Thanks to a further study of the characteristics of fluorescein angiography (FAG) in CSC, in 1967 Gass presented a more detailed view of the pathogenesis of the disease, as well as a clinical picture, and showed that CSC affects both the retina and the choroid. He introduced the new term idiopathic central serous choroidopathy [22]. With regard to the fact that the increased permeability of the choroidal vessels, leading to a seepage of fluid through the damaged RPE and subsequently to detachment of the neurosensory part of the retina, is generally acknowledged to be the cause of the pathology, the current nomenclature favors the term central serous chorioretinopathy [23].
In recent years, “multimodal imaging” has enjoyed a considerable expansion in the examination of chorioretinal pathologies. Thanks to modern examination techniques (“enhanced depth imaging” OCT, “swept-source” OCT, OCT-angiography), alongside polypoidal choroidal vasculopathy, pachychoroid neovasculopathy and pachychoroid pigment epitheliopathy, CSC is newly classified among “pachychoroid” diseases, a key feature of which is increased choroidal thickness (the Greek prefix “pachy” means thick) [24]. Another typical characteristic of CSC is dilation of the Haller’s layer of the choroidal blood vessels and compression of the choriocapillaris located above these vessels [25].
SYMPTOMS, DEMOGRAPHICS, RISK FACTORS
The pathology is characterized first of all by a slight blurring of vision, which is followed by varying degrees of deterioration of central visual acuity (VA) (6/6–6/60), micropsias, deformation of image, hypermetropia, a reduction of contrast sensitivity, change of color perception, and varying degrees of central scotoma. The symptoms tend to be mostly temporary and are often unilateral, although bilateral affliction is also relatively frequent [23]. It is not rare for CSC to have an asymptomatic course, or for serous retinal detachment to be identified entirely by chance during the treatment of a contralateral affliction of the eye [25]. The incidence of CSC varies according to different ethnic groups. A higher incidence has been recorded among Asians and whites in comparison with the black population. A bilateral and multifocal character of the pathology is also described more often among Asians [26]; high ablations of the RPE and neuroretina, which are more common in the Asian population, may be mistaken for Harada’s disease [27]. Men aged between 30 and 50 are predominantly affected (the average incidence of the disease in men and women is within a ratio of 8–9 : 1) [28,29]. In his study, Spaide stated the average age of patients with CSC at 51 years, although at the same time he conceded that in older patients, in whom the disease is more likely to be manifested in diffuse disintegration of the RPE or the presence of serous choroidal neovascularization (CNV), the initial signs of the disease may have appeared several years before the determination of the diagnosis [3]. Age-related macular degeneration (ARMD) associated with CNV may in some cases resemble CSC, and as a result in patients aged over 50 years it is necessary to exclude ARMD [30]. Despite the fact that the pathology is of an idiopathic character, it is known that CSC is generally more frequently associated with states of endogenous hypercortisolism (e.g. in Cushing’s syndrome, pregnancy) and in long-term systemic therapy with glucocorticoids (treatment of multiple sclerosis, asthma, Crohn’s disease), in which increased adrenergic stimulation of certain tissues takes place, influencing changes of blood pressure and tissue homeostasis [31–33]. In addition, a higher incidence of CSC has been described in women during the course of pregnancy (a condition with an increased level of freely circulating endogenous cortisol), in patients following an organ transplant with chronic use of immunosuppressants and corticoid therapy, or as a consequence of protracted stress burden [32,34,35]. Relatively substantial significance is attributed to psychosocial aspects. In 1927, Horniker was the first to identify a correlation between psychological agitation and an increased risk of the development of CSC; he referred to the pathology itself as retinitis centralis angioneurotica [36]. Nonetheless, it was not until 1986 that Yannuzzi definitively demonstrated an association between CSC and the type “A” personality (i.e. coronary personality with a tendency towards early onset of cardiovascular disease). This association is probably due to the increased level of catecholamines in circulation [37]. In Czech ophthalmological slang, CSC is referred to as the “managers’ disease”. According to other literary sources an increased incidence of the pathology has been described also in patients using phosphodiesterase type 5 inhibitors for erectile dysfunction [38], sympathomimetic substances such as pseudoephedrine, oxymetazoline, 3,4-methylenedioxy-N-methylamphetamine [39] or upon larger consumption of alcohol [35]. Mateo-Montoya and others demonstrated an increased incidence in patients with a positive test for Helicobacter pylori, the presence of which may also trigger extraintestinal pathologies including CSC (urease, which Helicobacter pylori produces, activates blood platelets, which in the region of the choroid may lead to vascular congestion and subsequently choroidal ischemia) [40,41]. Hypertension, obstructive sleep apnea, smoking, alcohol, antihistamines, and allergic respiratory disorders are further risk factors stated in the literature [42].
Although this concerns an acquired disorder, genetic predisposition plays an important role in the pathophysiology of CSC, as does genetic polymorphism [43]. A probable connection between the gene for complement factor H and CSC has been demonstrated by several studies independently of one another [44–46]. However, the precise mechanism of influence of factor H in the pathogenesis of CSC is still not known.
CSC may appear in 2 basic forms – acute and chronic. The designation of chronicity of the pathology has not been precisely defined by any authorities. Some authors define chronicity as persistent SRF for a period of 6 months [47], whereas recent clinical trials have inclined towards a duration of the disease for a minimum of 3 months [12]. In most cases of acute forms of CSC, spontaneous resorption of SRF takes place within 3 months of the onset of the pathology, with adjustment of visual functions with final central VA often of 0.8 and better (in 80–90% of cases) [48]. However, even despite the improvement of central VA, after the subsidence of the disease patients may complain of dyschromatopsia (impairment of color perception), reduced contrast sensitivity, metamorphopsia (perception of deformed image), central scotoma, and in rare cases night blindness. According to the results of the study conducted by Ooto et al., who examined the density of cones in the macular landscape in patients with resulting VA of 20/20 and better, even after the resorption of the SRF and upon preservation of the ellipsoid zone (EZ), there was a reduction in the density of cones in the originally affected region [49]. This data may explain the residual visual complaints even in patients with excellent resulting VA and complete anatomical adjustment. The determination of BCVA itself thus need not necessarily fully reflect the actual quality of visual functions in patients with CSC. CSC may lead to permanent damage to sight if the SRF is not absorbed, or if attacks are repeated and the condition progresses to the chronic stage. As many as 52% of patients recorded a recurrence of the pathology within 1 year of the first attack [50]. Long-term detachment of the neuroretina leads to cell death of photoreceptors, progression of RPE atrophy, subretinal fibrosis, the development of secondary CNV or cystoid macular degeneration, and thereby to a permanent deterioration of visual functions with central VA of 0.1 and worse. The final clinical picture is presented by DRPE, above which SRF may, though need not necessarily, be present [23].
PATHOPHYSIOLOGY OF THE DISEASE
The precise pathophysiology of the disease is not known in detail, and it is assumed that it incorporates a somewhat diverse etiology and mechanisms, which in their consequence lead to extensive abnormalities of choroid circulation and subsequent breach of the RPE [51]. Thanks to new imaging and examining techniques, a number of theories have emerged to explain the possible cause of the pathology; in their way each of them may contribute to explaining the onset and progression of the pathology at least in part. An insight into the issue of the pathophysiology of CSC and its dynamics is best provided by fluorescein and indocyanine green angiography. The anatomical relations of the eye in CSC are then best identified by OCT (especially in the case of enhanced depth imaging – EDI-OCT) and OCT-angiography – OCT-A).
Choroidal dysfunction theory
The current understanding of the pathogenesis of CSC places the greatest emphasis on the role of the choroid. Gass believed that the focal increase in permeability of the choriocapillaris was the primary cause of damage to the RPE layer situated above it, leading to detachment of the RPE, serous detachment of the neuroretina and in approximately 10–15% of patients to the formation of serofibrinous subretinal exudates [1]. The increase in the permeability of the choroidal vascular bed is probably caused by stasis in the vessels of the choroid, ischemia or inflammation, which is attested to by increased fluorescence of the inner choroid, especially in the central part of the ICG angiogram [3,52]. Guyer et al. proposed a potential model of the pathogenesis of CSC based on ICG-videoangiography (ICG-V). The ICG angiogram detected dilation of the large choroidal blood vessels and multifocal areas of hyperfluorescence in practically all the choroidal layers. The authors recorded diffuse zones of hyperpermeability within the surrounding area of active infiltrating points, which were evident on ICG-V, but not upon FAG examination. As a result, they concluded that hyperpermeability was on the level of the choroid, which can be better examined with the aid of ICG, rather than on the level of the RPE, the stability of which is more visible on FAG. Choroidal hyperpermeability increases hydrostatic pressure in the choroidal space, which then further increases demands on the RPE layer; this leads to its detachment, weakening, defect and eventual decompensation. This is then followed by infiltration, thus of the diffuse of fluid, electrolytes and proteins into the subretinal space, thereby causing detachment of the neurosensory part of the retina [53]. At the same time, breached cells of the RPE lose their capacity to pump fluid in a retrograde direction – thus from the subretinal space into the region of the choroid. RPE cells may in fact focally lose their polarity and actively pump fluid into the subretinal space [54]. This condition limits the absorption of SRF and is therefore decisive with regard to the size, shape or chronicity of serous detachment of the neuroretina and/or RPE. Spontaneous resorption of fluid may occur if the defect in the RPE heals, and if at the same time the function of the RPE is at least partially preserved. The cause of increased permeability is not entirely known, but it is assumed that stasis in the choroidal veins, choroidal ischemia or inflammation may play an important role [52,55,56]. In the study conducted by Prunte and Flammer, dilated capillaries and draining venules in one or more choroidal lobules followed by a localized delay in arterial filling may explain the cause of damage to the RPE layer above these areas of increased choroidal permeability. These conclusions support the idea of local lobular choroidal inflammation or ischemic choroiditis [52]. Abnormal regulation of blood flow of the choroid may be the main mechanism leading to typical “pachychoroid” changes [57]. The primary role of the choroid is newly supported by findings on EDI-OCT, demonstrating an increased thickness of this layer in patients with CSC [4].
From the above it ensures that intensive exudation of the choroidal channel in active CSC is caused primarily by breach of the vascular wall, abnormality or defect of the autoregulation of choroidal blood flow [58], and not the presence of CNV. There is strong evidence in favor of choroidal vasculopathy in the etiopathogenesis of CSC, though we still lack a precise delineation of the fundamental mechanism of origin of choroidal lesion.
RPE dysfunction occupies a significant role in the pathogenesis of CSC, and for this reason advocates of this theory prioritize defect on the level of the RPE as the primary cause of the onset of the pathology [59–61]. FAG in patients with active CSC detects one or more sources of leakage, causing serous detachment of the retinal pigment epithelium and/or neuroretina. The typical finding on FAG demonstrates fluid flowing into the subretinal space via a defect of tight connections between the cells of the retinal pigment epithelium. These focal leaking points through the RPE, which are typical of the acute form of CSC, were the first guidepost to determining the pathogenesis of the disease, and were considered the primary cause of accumulation of SRF [48]. Although it is rather the theory of choroidal dysfunction that is more widely recognized today, the RPE plays a fundamental role in the course of the pathology. Unlike the acute form of CSC, in which damage to one or more RPE cells occurs and serous detachment regresses spontaneously in most cases upon the expiration of these sources of seepage, in the chronic form, represented by diffuse disintegration of the RPE (which is clearly shown on autofluorescence and FAG), there is a widespread disruption of the RPE as the outer blood-ocular barrier and a defect of the capacity to pump fluid in a retino-choroidal direction, which leads to a chronic accumulation of fluid in the subretinal space and further damage to the RPE cells [23].
Combined choroid and RPE dysfunction theory
In probability, both theories contribute to explaining the cause and course of the pathology, namely increased permeability of choroidal vessels, elevation of hydrostatic pressure in the choroid and impaired function of the RPE [23].
Role of corticoids in the development of CSC
CSC induced by corticosteroids was first described in 1984 [62]. Since that time a series of clinical and experimental studies have been published, demonstrating that endogenous and exogenous corticosteroids contribute to the development of CSC [31,63–66]. Jampol et al. stated in their publication that corticosteroids might increase the sensitivity of choroidal blood vessels or RPE cells to the effect of endogenous catecholamines [67]. CSC is also more often identified in patients with a higher level of endogenous corticoids (Cushing’s syndrome, pregnancy, stress), similarly as in the case of patients with hypercortisolism resulting from of the treatment of ocular (uveitis, scleritis, optic neuritis) or systemic pathologies (bronchial asthma, hepatitis, allergic rhinosinusitis) [31–33,68–71]. Evidence exists attesting to the influence of glucocorticoids on the transcription and expression of adrenergic receptors, indicating the long-term effect of corticoids on the genome level, mediated by intracellular receptors. On the cellular level, the influence of glucocorticoids leads to increased expression of beta-adrenergic receptors [72]. On the molecular level, the same authors have demonstrated the effect of glucocorticoids on the expression of beta-adrenergic receptors through the presence of a higher level of intracellular receptor mRNA. Similarly, Sakaue also determined that expression and increase of the quantity of alpha-1ß-adrenergic receptors on the surface of the cells is induced by glucocorticoids [73]. The influence of expression on the genome level is not immediate, and is due to long-term exposure to higher levels of corticosteroids. The effect of corticoids on the non-genome level is prompt, and is determined by their direct interaction with the glucocorticoid receptors already present in the cell membranes; these incorporate ion channels or receptors for neurotransmitters [67,74]. Their activation causes damage to the endothelial cells of the choriocapillaris, and therefore its greater permeability and the degeneration of the RPE layer situated above it [37,75].
Pathophysiology of loss of VA in CSC
Weakening of the foveal landscape, cystoid macular degeneration, and damage to the photoreceptors of the macular areaare considered reasons for the deterioration of VA in CSC [76,77]. Cystoid macular degeneration was first described by Iida as a cystoid intraretinal cavity without evidence of leakage on FAG [76]. A duration of the pathology of at least 5 years and subretinal fibrosis are considered the main risk factors [77]. The key factor regarding the resulting visual functions is the preservation of the outer retinal layers. Loss of photoreceptors and RPE cells upon a background of long-term detachment of the neuroretina leads to a significant deterioration of visual functions [78].
The aim of any classification of a pathology is to divide the broad range of manifestations of the pathology into subcategories, which assist us in determining its probable development, prognosis, and the most appropriate plan of treatment. To date several classifications of CSC have been published, but none of them have fully contained all possible manifestations of the disease. In very simple terms, up to now the pathology has been divided into the acute and chronic forms [23]. Daruich et al. recently proposed a new classification, which incorporates the individual (and rare) manifestations of CSC [79], and this classification is now generally recognized [80]:
1) Acute CSC: The most common type, predominantly affecting younger patients and causing acute localized detachment of the RPE and/or neuroretina with mild to medium deterioration of visual functions. On FAG we find one or more sources of dye leakage. In 95% of cases, spontaneous resorption of the SRF takes place, as well as adjustment of visual functions, though in certain patients a disorder of color perception may persist [81].
2) Persistent CSC: In a certain percentage of cases, SRF persists for longer than 3 months. As a rule, for these patients we indicate new FAG/ICG in order to identify the locations of leakage. The result of angiography may be hyperfluorescence in the original location, a new source of leakage or no demonstrated activity. If a new source of fluorescein leakage is demonstrated, the pathology is considered persistent.
3) Recurrent CSC: Defined as the repeated presence of SRF following its documented complete reabsorption, occurring in 50% of patients in the first year of the disease [82]. On FAG we may find the same source of leakage or a de novo appearing hot-spot.
4) Chronic CSC: Serous elevation of the RPE and/or neuroretina persisting for longer than 3 months upon a background of chronic changes and alteration of the function of the RPE. Long-term detachment of the neurosensory retina leads to a further worsening of of the RPE alteration, the occurrence of typical widespread atrophic traces of the RPE and atrophy of the photoreceptors [83]. In its advanced stage, this variant is also referred to as DRPE, and is typified by a varying scope of atrophic RPE lesions, with or without detachment of the neuroretina [84].
5) Inactive CSC: Cases with a medical history of CSC episodes, though currently without signs of SRF.
In addition to the above basic types, there is also a smaller group of patients who have the following atypical clinical picture in CSC:
Atypical CSC: In advanced chronic forms (persisting for several years), in addition to persistent serous ablation of the neuroretina and multiple atrophic RPE lesions, there may also be presence of cystoid macular degeneration [76], multifocal CSC [85] or flat irregular ablation of the RPE (“wavy RPE”), beneath which there may be presence of incipient secondary type I CNV [86,87]. CNV upon a background of CSC is generally not entirely clearly demonstrated on FAG, and in many cases also not on ICG, though it may now be detected with a high degree of sensitivity and specificity with the aid of OCT-A [88,89].
Bullous CSC: A rare clinical variant of chronic CSC is bullous serous retinal detachment (Fig. 1). Due to the influence of gravity, the maximum SRF tends to be located in the inferior quadrants, and in some cases we may find massive deposits of subretinal fibrin [90].
1. Color fundus photo, optical coherence tomography scan and fluorescein angiography (Visucam Zeiss, Heidelberg Spectralis, Myslík Manethová) 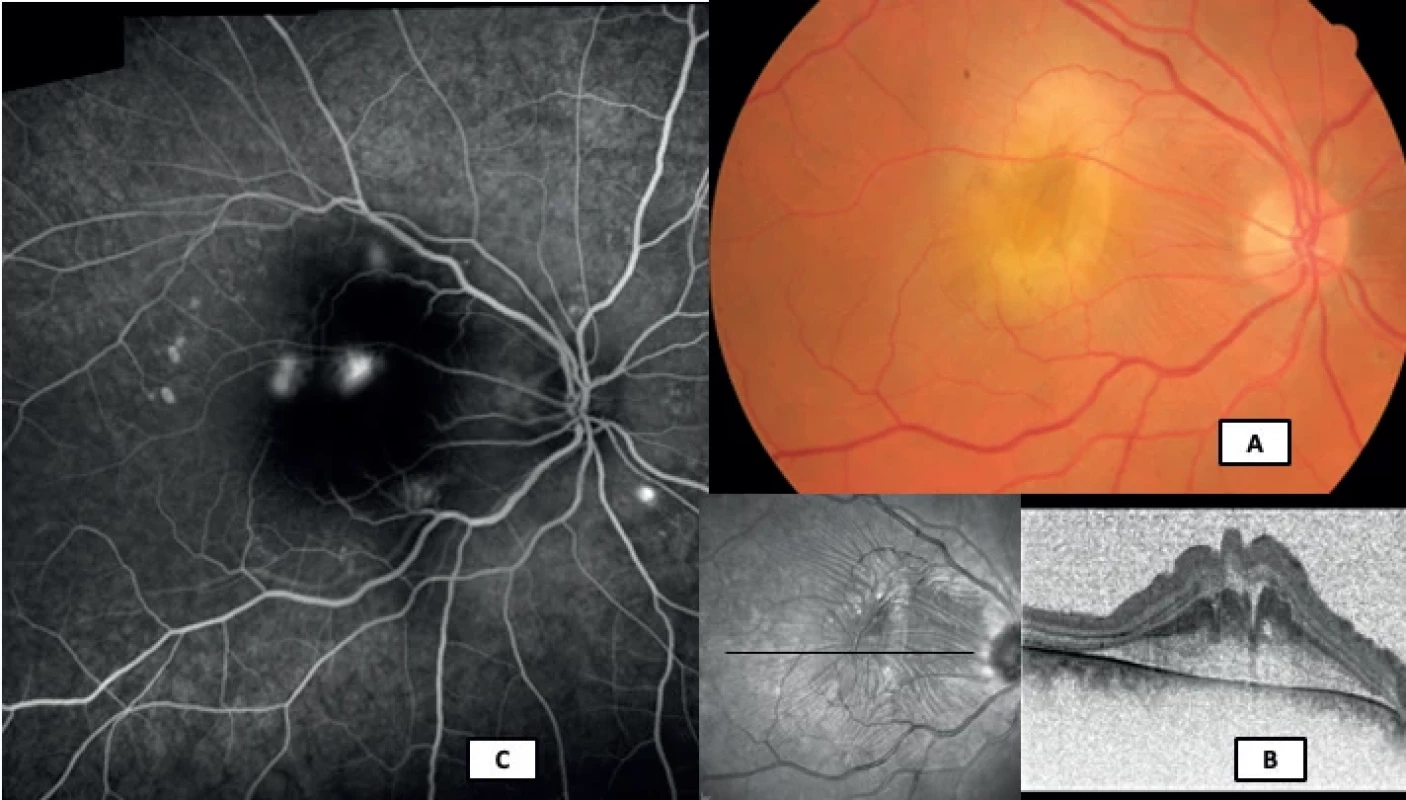
in a 38-year-old patient with an atypical – bullous – form of central serous chorioretinopathy: (A) color image of excessive yellowish subretinal fibrin exudation in center of the macula with radial
retinal folds, (B) transfoveolar linear scan with a subretinal hyperreflective mass and significant destratification of the
inner retinal layers, (C) wide-field fluorescence angiography with multiple active hot-spots of varying intensityExamination of distance visual acuity
Examination of VA ranks among the fundamental and most important functional ocular examinations. In regular practice Snellen charts are most commonly used, though these do not enable standardized examination, the change of letter size between rows is not regularly progressive, and the letters have varying degrees of difficulty of legibility. A more precise determination of VA is attained by the use of ETDRS optotypes. Their advantage is the same number of letters in the row, the same degree of legibility of the individual characters, in which they maintain geometric progression of the size of optotypes in individual rows. Repeated testing demonstrates very positive results in comparison with the Snellen method, and this can be used in clinical research, providing more detailed (sensitive) changes of values of visual acuity in the observed period.
Biomicroscopic examination of the ocular fundus
In biomicroscopic examination of a patient with CSC, we detect typical detachment of the neuroretina, which is evident as a sharply bordered, transparent “blister” on the posterior pole of the eye. As a rule, the foveal reflex is not generally evident, and is rather replaced by a “halo effect” resulting from the reflection of the light from the detached neuroretina. In the place of detachment, the retina is of normal thickness, without any change in transparency. The fovea may manifest a slightly yellowish color in the biomicroscopic image due to the visible xanthophyll of the central part of the retina. Numerous yellowish-white fibrin deposits may be present, covering the posterior surface of the elevated neuroretina [23]. Typical manifestations are irregularities of the RPE, which are accentuated with chronicity of the condition. Upon rigorous biomicroscopy (subsequently verified with the aid of OCT) we may find one or more loci of serous detachment of the RPE. In the case of a long duration of the disease, the subretinal fluid may stimulate fibrotic changes, which may lead to permanent functional changes. However, in the majority of cases a gradual resorption of fibrin deposits takes place. Conditions predisposing to exudation of fibrin are extensive or numerous PEDs (RPE ablations), chronic or recurrent form of the pathology, systemic treatment with corticosteroids, pregnancy and organ transplantation [23].
Excessive exudation may lead as far as bullous serous retinal detachment, with maximum in the inferior quadrants [90]. Chronic forms are typified rather by flat serous separation of the neuroepithelium above areas of the dystrophic altered RPE, which in places may progress as far as flat atrophy. These atrophies are most commonly found in the macular and peripapillary zone, and “trickle” downwards due to the influence of gravity upon chronic exudation (Fig. 2) [91].
2. Fluorescein angiography of a 54-year-old patient with a chronic form of central serous chorioretinopathy (Heidelberg Spectralis, Myslík Manethová): 
late venous phase of wide-field angiography showing a wide area of diffuse disintegration of the retinal pigment epithelium layer and a typical gravity trace above the terrain of dystrophically altered retinal pigment epithelium after long-term subretinal exudation Instruments enabling the recording of an image of the retina on classical or digital photography are founded on a similar imaging principle as indirect ophthalmoscopy. A photograph of the ocular fundus is appropriate for evaluating the condition, monitoring the progression and effect of treatment of the pathology. At present the method is being progressively replaced by techniques which identify the current clinical finding and its development over time in greater detail (OCT, FAG/ICG, OCT-A). Nevertheless, color photography of the ocular fundus retains its place especially in the photographic documentation of atypical or rare forms of CSC.
We consider fluorescein angiography to be the oldest and also the fundamental examination technique for determining the diagnosis, evaluating the finding and excluding other differential diagnoses in CSC. FAG is an invasive imaging method, which serves to examine the flow of blood in particular through the retinal and to a lesser degree also choroidal vascular bed, with the use of a contrast substance applied intravenously (fluorescein sodium salt). The examination provides dynamic information about the condition of the inner and outer blood-ocular barrier, and partially also about the condition of the RPE layer.
A characteristic finding in the early phase of the angiogram is the presence of one or several sources of fluorescein leakage on the level of the defective RPE, with progressively increasing fluorescence and scope into the late venous phase, referred to as “inkblot”. In approximately 10% of cases a typical “smokestack” formation is visible, in which the dye gradually diffuses into the space of serous detachment of the neuroretina, thereby creating an image similar to rising “smoke from a smokestack” [23].
In chronic forms of the disease, it may be the case that no unambiguous point sources of hyperfluorescence are identified, but rather areas with a diffuse window defect in the early phases, and increased diffused fluorescence in the late phases of the angiogram, which are evident in diffuse atrophic changes of the RPE in the chronic stages of the pathology. According to the character of the finding and the dynamic changes during the course of the angiogram, it is possible to assess the degree of activity of the pathology and thus to select an adequate therapeutic approach [48,92–94]. Cystoid macular degeneration or CNV upon a background of CSC may not necessarily be entirely demonstrable on FAG, and it is therefore always a benefit to use multimodal imaging due to the greater probability of identifying all the abnormalities and determining the correct diagnosis [76,89].
FAG is considered the fundamental examination method for this pathology, and serves as a template for any applicable treatment of the retina by a laser coagulation beam in the place of point hyperfluorescence recorded in the early phase of the angiogram, or by a thermal laser stimulation beam in photodynamic therapy in the place of diffused hyperfluorescence in the late phases of the angiogram. FAG is also beneficial in differential diagnostics, and enables the exclusion of pathologies which have a similar clinical picture to CSC (Vogt-Koyanagi-Harada syndrome, CNV upon a background of ARMD, infectious disease of the posterior segment of the eye etc.).
Indocyanine green angiography (ICG)
ICG angiography has expanded our knowledge about the pathogenesis of this disease. Thanks to its properties, it detects particularly deeper layers – the choroid – thus the place of origin of the pathology. The principle of examination is identical to the procedure upon fluorescein angiography, with the only difference that the dye used is indocyanine green, which binds very quickly to blood plasma proteins (98%). After exposure through excitation light, ICG emits a fluorescent light with a wavelength within the range of 805 – 835 nm, which explains the relatively high transmission through the ocular tissue (xanthophyll and melanin of the RPE, opalescent lens, choroid). For this reason, ICG angiography is appropriate for visualization of the choroidal vascular network, and therefore offers a precise evaluation of the extent of the choroidal lesion in patients with CSC [48,55,56]. In the early phase of the angiogram, as a rule a delay appears in the filling of the choroid; early hyperfluorescence attests to choriocapillaris nonperfusion. This leads to dilation of the choroidal veins, which in the mid phase of the angiogram is displayed as geographical area of increased fluorescence due to hyperpermeability, especially of the large choroidal vessels, known as the Haller’s layer. Areas of hyperfluorescence expand centrifugally into the venous phase, and may not be strictly localized only in places corresponding to places of hyperfluorescence visible upon FAG examination (on the contrary, very often they may be observed in places which appear to be clinically normal, or in the contralateral, unaffected eye). In the late venous phase, the dye is either washed out of the tissue or hyperfluorescence persists, which among other factors depends on the degree of activity of the pathology [48,55,92–95]. An unequivocal advantage is the performance of simultaneous FAG and ICG, with a mutual comparison thereof. Choroidal vascular hyperpermeability is a common feature of all forms of CSC [94,95]. However, the areas of hyperfluorescence (activity) in FAG very often do not entirely correspond to the areas of increased activity demonstrated with the aid of ICG (Fig. 3). For this reason, it may be possible for us to determine the development of activity better through a mutual comparison than by performing the methods separately.
ICG imaging is an essential examination method before planned PDT, and provides us with the entirely exact scope, frequency and localization of the choroidal lesion, which may not always be visible on the FAG image. Similarly, ICG has an irreplaceable role in cases of unsatisfactory response to treatment or if CSC has a recurrent course, in which other choroidal lesions may be activated over the course of time [96].
3. Simultaneous fluorescein and indocyanine green angiographic image in a 58-year-old patient with a multifocal chronic form of central serous chorioretinopathy (Heidelberg Spectralis, Myslík Manethová): 
the middle phase of the angiogram with increased fluorescence of the pathologically changed retinal pigment epithelium at the upper temporal arcade (red asterisk); on indocyanine green angiography, 3 significantly dilated and leaking large choroidal vessels of Haller's layer are visible in the inferior temporal arcade, but they are not visible on fluorescein angiography (yellow arrows) Thanks to spectral domain OCT (SD-OCT) and more recently also thanks to enhanced depth imaging OCT (EDI-OCT) and en face swept-source OCT (SS-OCT), which enable the imaging of retinochoroidal tissue throughout its entire thickness, we are now better able to understand the anatomy and partially also the pathophysiology of CSC. OCT is a contactless, noninvasive, nonmydriatic, safe, fast and effective examination method, which is used as the method of first choice for the quantitative evaluation of CSC, monitoring of the development and effect of treatment. It is a highly specialized examination of the macula on the principle of low-coherence interferometry with high resolution, which in the form of an incision enables the imaging of not only retinal but also choroidal structures, with a high-resolution capacity of up to 3 µm [97]. Thanks to its high-resolution capacity it provides detailed information about the structures on the level of the vitreoretinal interface, the individual layers of the retina, RPE, Bruch’s membrane, and by means of current options also the individual layers of the choroid. A typical finding on OCT in CSC is the presence of hyporeflective (non-reflective) fluid beneath the layer of the RPE and neuroepithelium. Elongation and thickening of the outer segments of the photoreceptors occur in the place of serous detachment of the neuroretina, and in places also their erosion, ensuing as an abrasion mechanism upon pronounced exudative activity above the RPE defect [79]. In 50–100% of cases of CSC, RPE ablation is present [98,99]. Variously high RPE ablations of a serous character are typical of early forms of the disease, and appear most frequently above places of increased choroidal permeability [79]. In the case of chronic lesions, we rather observe irregular flat undulation of the RPE layer, and the presence of dense (hypo - and hyperreflective) material between the Bruch’s membrane and the RPE, which on OCT imaging creates a “double layer sign” [99,100]. Another sign of rather chronic lesions is hyperreflective foci visible in the retinal layers, in the subretinal space and in the region of the inner choroid, which correspond with hyperautofluorescent deposits evident on the retina [101–103]. It appears that a greater quantity of hyperreflective foci correlates with a longer duration of the disease and a worse prognosis of resulting VA [104]. In the case of recurrent or several-year duration of the pathology, we may often find cystoid macular degeneration (CMD) on OCT scans, as well as thinning of the outer nuclear layer (ONL) of the retina, disruption of the ellipsoid zone (the junction between the outer and inner segments of the photoreceptors) or the external limiting membrane (ELM), an absence of photoreceptors or general atrophy of the retinal layers [102].
In comparison with the healthy population, EDI-OCT demonstrated a generally greater thickness of the choroid and larger choroidal vessels of the Haller’s layer in patients with CSC, as well as conversely thinning of the Sattler’s layer together with the choriocapillaris in both the affected and the contralateral eye; this is probably caused both by the pressure of dilated vessels and by primary atrophy of the choriocapillaris upon hypoperfusion of the tissue [99,105]. Change of choroidal thickness may be focal or diffuse, and may not necessarily entirely correlate with areas of hyperfluorescence on ICG in the places of vascular hyperpermeability of the Haller’s layer [106]. These findings further confirm the idea that CSC may be caused by locally increased hydrostatic pressure of the choroid [4,28,105,107]. With regard to the thickness of the choroid, according to the current nomenclature CSC is classified among pachychoroid diseases (together with pachychoroid pigment epitheliopathy, pachychoroid neovasculopathy, polypoidal choroidal vasculopathy, and focal choroidal excavation) [24] (Fig. 4).
4. Combined image of fluorescein angiography and transfoveolar linear scan of optical coherence tomography (Heidelberg Spectralis, Myslík Manethová) 
of a 48-year-old patient with chronic long-lasting central serous chorioretinopathy
with typical elongation and thickening of photoreceptors, hyperreflective dots present (red asterisk),
preserved external limiting membrane (green arrow), marked by noticeably dilated lobular choroid (yellow) and focal
abrasion of photoreceptors in the maculopapillary bundle (purple asterisk)Optical coherence tomography angiography
OCT-A is a new, promising, noninvasive method, with the aid of which it is possible to examine the retinal and choroidal vascular network without the use of an intravenous contrast substance, with high-resolution capacity in all layers of the retina and choroid. The principle of OCT-A is to detect the movement of particles; on the assumption that the only moving particles in an otherwise motionless eye are blood elements (erythrocytes). It therefore concerns the recording of blood flow in the vessels of the retina and choroid. OCT-A images may be evaluated summarily or in isolation in the individual vascular layers, which enables a very detailed analysis of the vasculature on various levels without blurring of the structures, as tends to be the case upon leakage or accumulation of dye in classic FAG [89]. On OCT-A images we usually detect abnormal flow through the large, dilated vessels of the choroid and the dilated vessels of the choriocapillaris. An abnormal flow of the choriocapillaris, which is manifested as a dark zone on the image, attest to a reduction of flow correlating with the finding on ICG in acute, but especially in chronic forms [108].
Costanzo et al. described 3 specific findings on OCT-A in the region of the choriocapillaris: dark areas, dark points and abnormal choroidal vessels. The dark areas are described as diffuse or focal, rough, imprecisely bordered regions with a low or undetectable flow, whereas the dark points are black, individual or multiple sharply bordered deposits without any detectable blood flow on the level of the choriocapillaris. The dark areas mostly correspond to the zones of SRF, whereas the dark points correspond with ablations of the RPE. Abnormal choroidal vessels are described as manifest, clearly delineated, intricate dilated vessels with a large flow. The interpretation of these abnormal choroidal vessels should be conducted with caution, because in many cases it is not easy to differentiate them from the choroidal neovascular network [89,109]. Furthermore, today OCT-A far better enables us to detect the presence of secondary CNV, usually appearing beneath an irregularly undulating RPE, which is not very often detectable on FAG or ICG examination [87,89,110,111]. OCT-A is thus becoming an entirely revolutionary imaging method, which provides us not only with relatively exact information about the structure, but also indirectly about the function of the retinochoroidal tissue, and should therefore always be included upon the examination especially of chronic forms of CSC.
Fundus autofluorescence (FAF)
Autofluorescence, a noninvasive imaging method, has gained substantial popularity in the last decade. It is the only examination technique to enable the detection of the metabolic activity of physiologically or pathologically present fluorophores of the retina in vivo (e.g. lipofuscin granules, collagen, elastin, fibrin), thus structures distinguished by varying degrees of autofluorescence. During the examination 2 types of FAF examination may be applied: short-wave FAF (SW-FAF) or near-infrared FAF (NIR-FAF). SW-FAF is irradiated by lipofuscin granules of the RPE and thereby provides information about the condition of the RPE. NIR-FAF originates from melanin pigment of the choroid and RPE. Although NIR-FAF is used less frequently, clinical trials have demonstrated that in the case of CSC it may be far more sensitive in detecting changes of the outer retinal layers [112,113].
Physiological FAF is typified by dark imaging of the optic nerve and vessels due to the absence of autofluorescent fluorophores and homogenous autofluorescence of the RPE [93,114,115]. In eyes with acute CSC there is a characteristic mottled image of increased autofluorescence in the zone of the ablated sensory epithelium due to the present of subretinal or intraretinal precipitates and deposits with an increased lipofuscin content (accumulation of the outer segments of the photoreceptors, which are not phagocyted by the breached RPE, or an accumulation of macrophages with phagocyted outer segments) [114]. In the early phase, areas of hypoautofluorescence are evident, corresponding with locations of leakage in 70–100% of cases, thus in the place of the defective RPE [116,117].
In chronic forms, as in acute forms, the majority of eyes manifest hypoautofluorescence in the place of leakage visible on FAG, which is due to the presence of subretinal fluid and varying degrees of damage to the RPE. The pathognomic finding in long-term states is mixed autofluorescence of varying intensity. Especially the zones above the dystrophic altered RPE are hypoautofluorescent, while there is an absence of autofluorescence in places of RPE atrophy. At the outset these are generally hyperautofluorescent if subretinal fluid is present, but they progressively become hypoautofluorescent upon the gradual degradation of the RPE cells, up to their complete atrophy. Studies have demonstrated a relatively good correlation between the findings on FAF and retinal sensitivity quantified by microperimetry and VA, and FAF may therefore be used in order to estimate the degree of functional changes in eyes with CSC, as well as for the prognosis of the change of BCVA in connection with the therapeutic intervention [117].
5. Transfoveolar linear optical coherence tomography scan (Heidelberg Spectralis, Myslík Manethová) of a 61-year-old patient with chronic central serous chorioretinopathy showing: 
(A) flat serous detachment of neurosensory retina in the area of
the central macula; at the edge of the detachment, the line of photoreceptors is still preserved (red arrow), while subfoveolarly,
the line of photoreceptors is already absent due to the long-term presence of subretinal fluid (yellow arrow). (B) depicts the state
after a photodynamic therapy with half the dose of verteporfin with a satisfactory anatomical effect, however, the transfoveolar
linear scan reveals total retinal atrophy in the central part of the macula with atrophy of the subfoveolar retinal pigment epithelium
layer above it (blue arrow) with a noticeable absence of the photoreceptor line (yellow arrow); and vice versa by a relatively
well-preserved interdigitation zone at the edge of the original neuroretinal detachment (red arrow)Macular microperimetry detects reduced macular sensitivity in patients with CSC, and may be a useful subjective examination method for evaluating response to photodynamic therapy. The results of clinical trials indicated that mere examination of VA alone may significantly underestimate changes to macular functions, which accompany successful treatment of CSC. Macular microperimetry has been demonstrated to be an appropriate method for precisely evaluating the sensitivity of the retina in patients treated with the aid of PDT or micropulse laser, in whom better sensitivity has been demonstrated in the central retinal region after therapy [117,118].
Multifocal electroretinography
Multifocal electroretinography (mfERG) serves for objective evaluation of functional changes of the retina in patients with CSC. Improvement of BCVA and central retinal thickness following therapy with the aid of PDT was in correlation with response to mfERG; although after absorption of the subretinal fluid there was a significant improvement in the recording on mfERG, the original values are not generally attained [119,120].
In the majority of cases, the results of laboratory examinations are not of any benefit for determination of the diagnosis. Nevertheless, in recent years a correlation has been stated with increased serum levels of plasminogen activator inhibitor 1 in patients with CSC [35]. Higher values of endogenous cortisol may also be identified, which is considered one of the main risk factors of the onset of CSC, or abnormal values of blood coagulation parameters (e.g. lower level of growth factor from blood platelets) [79,121]. However, no laboratory test exists which is specific for the diagnosis of this pathology.
THERAPY OF CSC
Even despite all the advances in diagnostics, examination and therapeutic options, the optimal approach and choice of adequate treatment of CSC remains contentious. This controversy is due to the fact that although the pathology has a substantial tendency towards spontaneous recovery, with an excellent prognosis of resulting VA, on the other hand long-term retinal detachment may lead to pronounced irreversible anatomical and functional changes (Fig. 5). The principle of treatment is to induce reattachment of the neuroretina, to improve or preserve VA and to minimize the risk of recurrence. Upon selection of a suitable method of treatment it is always necessary to take into consideration the potential temporary course of the disease, the localization of the pathological leakage and the condition of the RPE.
Conservative approach – observation and modification of risk factors
In the case of the acute phase of the pathology, especially if serous detachment of the neuroretina is caused by a specific source of leakage, spontaneous resorption of the SRF occurs in the majority of cases (80–90%) within 3 months of the onset of the disease [47,122]. As a result, in the acute phase (first 3 months of the pathology), the recommendation is rather to observe the condition and attempt to reduce risk factors. It is appropriate to reduce or completely discontinue corticoid therapy if the patient uses this therapy chronically, and to adjust the patient’s lifestyle, minimizing stress stimuli, and if applicable also to administer conservative therapy. However, situations exist in which therapy should be applied earlier. These are for example when the patient has high demands for improvement of vision (drivers by occupation, graphic artists etc.), recurrent form of the disease or in the case that untreated CSC in the contralateral eye has led to a significant deterioration of vision.
Although a range of studies have been conducted to examine the effect of orally administered pharmaceuticals, no causal medication exists at the present time. No positive influence has been demonstrated in the case of beta-blockers, anxiolytics, antidepressants, non-steroid antiphlogistic drugs, resorptive drugs, vasoprotectives or vitamin therapy [123]. Corticosteroids previously used as standard for the conservative treatment of CSC are no longer recommended whatsoever, since in the literature there are a whole range of studies indicating that general and parabulbar administration of corticoids may provoke the onset of CSC [35,66]. Long-term corticoid therapy is linked to prolonged presence of SRF and diffuse changes of the RPE, and in patients who for whatever reason are unable to reduce their dose of corticoids, we frequently struggle with a protracted, chronic pathology that responds to treatment only with difficulty. Care for these patients requires interdisciplinary cooperation, and the recommendation of an ophthalmologist for patients with CSC whose condition of health so permits is to discontinue long-term corticoid therapy or replace it with alternative treatment (e.g. biological therapy). Alkin et al. present the case of a positive effect of nepafenac 0.1% in the treatment of acute forms of CSC [124]. New observations have appeared in the literature attesting to the positive effect of mineralocorticoid receptor antagonists, especially eplerenone. According to recent results of clinical trials, eplerenone influences a number of pathophysiological pathways, thereby supporting the more rapid absorption of fluid, and is more effective in chronic states [125,126]. In the case of extensive RPE atrophies, however, its effectiveness is limited [127]. Further prospective clinical trials are essential in order to confirm its safety and efficacy, and to determine which clinical forms of CSC would profit from treatment with eplerenone.
Conventional argon laser therapy has been used for several years in order to manage both the acute and chronic phase of CSC [16]. With regard to the large percentage of spontaneous resorption of SRF, the recommendation is to wait 3 months from the onset of the pathology before intervention [128]. If spontaneous reattachment of the neuroretina does not take place and the point of leakage is sufficiently distant from the center of the fovea (more than 500 µm), direct laser photocoagulation of the leaking point is indicated (hyperfluorescence according to FAG). If the hot-spot is less than 500 µm from the center of the fovea, or if diffuse affliction of the RPE is already expressed, the recommendation is to select an alternative therapeutic approach [128]. Following laser coagulation of the hot-spot, in uncomplicated cases anatomical reattachment of the neuroretina usually takes place within 2 weeks of the performance of the procedure. Adjustment of central VA as a rule requires twice that amount of time. Photocoagulation usually reduces the period of duration of the pathology and accelerates resorption of the SRF, but has no influence on resulting VA [129].
The aim of laser photocoagulation is the scarring of the defective RPE through which fluid is penetrating into the subretinal space. Targeted point laser coagulation of the hot-spot is performed on the basis of prior fluorescein (or indocyanine green) angiography. The principle of the dual healing effect of the photocoagulation laser is a direct thermic effect, as well as secondary expansion of the RPE cells from the surrounding healthy tissue and healing of the RPE defect [130]. In the place of application of the laser beam, the thermal destruction of tissue and the adjacent neurosensory retina takes place, and absolute scotoma may appear in the place of treatment. Potential adverse effects of laser photocoagulation should be taken into consideration, especially if it is necessary to use a laser in close proximity to the fovea or in the maculopapilar bundle. Further potential complications include accidental affliction of the fovea, hemorrhage, fibrosis, secondarily occurring CNV, slow progressive enlargement of the place of RPE atrophy of the original laser beam (enlargement of the laser spot may in time affect the fovea and cause irreversible loss of VA) [16].
A modality to a conventional laser is a subthreshold or micropulse laser. The principle of a micropulse laser is the division of the continual laser beam of a wavelength of 810 nm (or 577 nm) into individual micropulses separated by a pause, thus minimizing the generation of thermal energy that has a mutilating effect on the tissue in the treated and surrounding area. It supports the biological response of the tissue, which leads to a restoration of the integrity and physiological function of the RPE cells, with resorption of the SRF. Subthreshold diode micropulse laser therapy may be a safe and effective therapeutic modality for cCSC in future [118].
In addition to laser therapy, photodynamic therapy has also been used successfully in recent years. It was introduced into practice based on the idea that CSC primarily affects the choroid. PDT was originally used in the treatment of solid tumors, and later in the treatment of wet form ARMD [131]. Positive results of treatment of chronic form of CSC by photodynamic therapy with the use of the photosensitizing substance verteporfin were first published by Yannuzzi and Piccolino in 2003 [77,132]. Since that time, the safety and effectiveness of ICG-navigated PDT in the treatment of CSC has been confirmed by a series of clinical trials [11,13,135–136].
The principle of PDT consists in the intravenous administration of the photosensitizing substance verteporfin (Visudyne, Novartis AG, Bülach, Switzerland), and its activation through the use of laser light in red wavelengths. Because the induction of the photochemical reaction takes place intravenously, there is no thermal damage to the tissue during PDT. In the place of irradiation, PDT induces constriction of the choroidal vessels, leading to their modification, reduces choroidal perfusion, and as a consequence of these changes reduces choroidal exudation [137,138]. According to the conclusions of clinical trials, PDT enables more rapid resorption of the SRF in comparison with the group of patients who are treated with laser photocoagulation [132,139]. In order to determine the precise localization and extent of the lesion, the results of ICG (medium venous phase) are used, according to which the laser spot is targeted precisely at the zone(s) of hyperfluorescence during the treatment [94,140]. PDT may be applied in the standard dose and length of irradiation of the lesion, which is used in the treatment of wet form ARMD (parameters according to TAP study: laser beam with a wavelength of 689 nm applied for a period of 83 seconds in exposure of 600 mW/cm², within 5 minutes of the end of intravenous infusion of verteporfin – in a dose of 6 mg/m², energy 50 J/cm²) [137]. With regard to the potential danger posed by standard PDT treatment (deterioration of VA, wider destruction of normal or already breached RPE with atrophic changes, potential choroidal ischemia, risk of development of secondary CNV), in recent years priority has been given to alternative dosing regimens of verteporfin and the used laser beam, known as “safety-enhanced” PDT. Either a reduced dose of radiation is used in “reduced (half)-fluence” (HF-PDT – 42 seconds, or half energy – 25 J/cm2), or a reduced dose of verteporfin in “reduced (half)-dose” (HD-PDT, i.e. 3 mg/m² of Visudyne) [12,28,128,133,141].
The results of studies comparing “safety-enhanced” PDT and conventional PDT are comparable. While achieving a similar anatomical and functional effect, “safety-enhanced” PDT minimizes the harmful effect on the perfusion of the choriocapillaris and its atrophying influence on the RPE and thickness of the retina [12,133]. The authors Nicoló and Kim in their publications compared the functional and anatomical results in patients with cCSC treated using HF-PDT and HD-PDT. Both authors present findings that no statistically significant differences exist in the anatomical or functional results between these therapeutic modalities, Nicoló merely states more rapid absorption of SRF in the case of HD-PDT [10,141].
Thanks to its positive results and satisfactory safety profile as demonstrated by the clinical trials conducted to date, reduced PDT is considered the treatment of first choice, although it remains off-label in indication for CSC [142,143].
Anti-VEGF therapy
With regard to the pathophysiology of the disease, the use of anti-VEGF agents is contentious in the treatment of CSC. A series of clinical trials have provided ambiguous results of the effect of anti-VEGF agents in the treatment of CSC [18,144,145]. The pathology itself is not associated with a higher level of VEGF in the vitreous, and the effect of anti-VEGF treatment for CSC without the presence of CNV has not been demonstrated overall [146]. However, a different situation applies in complicated cases of secondary CNV upon a background of CSC. In the last 5 years especially, when thanks to advances in multimodal imaging (OCT-A) it has become possible to demonstrate CNV associated with CSC pathology with far greater sensitivity and specificity, the use of anti-VEGF agents in these cases has gained ever-increasing significance, and in the most severe conditions in combination with PDT [147]. Previous clinical trials have demonstrated an anatomical and functional effect of bevacizumab, ranibizumab and aflibercept in the treatment of CNV associated with CSC [146,148,149]. However, only limited data exists documenting the safety and efficacy of combined PDT and anti-VEGF treatment. Asahi demonstrated positive results of combined therapy with HD-PDT + aflibercept in patients who were unresponsive to conventional treatment (laser photocoagulation, monotherapy with PDT, monotherapy with anti-VEGF), and recorded greater success above all in eyes complicated by CNV [150].
Ranibizumab is the only registered pharmaceutical in the Czech Republic that may be used in the treatment of rare forms of CNV, thus also those upon a background of CSC.
Other therapeutic alternatives
Despite the fact that no causal medicamentous therapy exists in the treatment of CSC, a series of smaller clinical trials have demonstrated positive results in the treatment of CSC upon the use of finasteride (dihydrotestosterone synthesis inhibitor) in a dose of 5mg per day or acetylsalicylic acid acting against choroidal vascular congestion, ischemia and inflammatory changes accompanying CSC [28]. Good results may also be attained by eradicating infection of Helicobacter pylori in indicated cases and treatment of obstructive sleep apnea as potential risk factors triggering this disease [28,35].
CONCLUSION
Today’s technical advances in the diagnosis and treatment of CSC in combination with our own clinical experiences represent a substantial improvement in the prognosis for our patients with CSC, for whom it is necessary to ensure not only correct diagnosis of the pathology based upon multimodal imaging, but above all correct setting and timing of adequate treatment. From our results to date it also ensues that treatment of chronic forms of CSC should be commenced in the early stages in order for us to attain the best possible anatomical and functional results, before irreversible changes are expressed such as DRPE or complicating CNV.
The author of the study declares that no conflict of interests exists in the compilation, theme and subsequent publication of this professional article, and that it is not supported by any pharmaceuticals company. The study has not been submitted to in any other journal or published elsewhere, with the exception of congress abstracts and recommended procedures.
MUDr. Kateřina Myslík Manethová, Ph.D., FEBO
Vídeňská 800
140 00 Praha 4 – Krč
E-mail: katerina.manethova@ftn.czReceived: October 2, 2022
Accepted: April 3, 2023
Sources
1. Atlas of Macular Diseases: Gass JDM. Stereoscopic Diagnosis and Treatment. 4th edition: Mosby, 1997.1061,52-70. ISBN: 978-0815134169.
14. Chan WM, Lai TY, Lai RY, et al. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28(1):85-93.
18. Lim SJ, Roh MI, Kwon OW, Woong OH. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010;30(1):100-106.
20. Bennet G. Central serous retinopathy. Br J Ophthalmol. 1955;39 : 605-618.
21. Maumenee AE. Macular Diseases: Clinical Manifestations. Trans Am Acad Ophthalmol Otolaryngol. 1965;69 : 605-613.
22. Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium: II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967;63 : 587-615.
23. Ryan SJ. Retina. Volume II, 4th edition, Baltimore (USA): Elsevier Mosby, 2006. 1889, 1135-1161. ISBN: 9780323025980.
24. Gallego-Pinazo R, Dolz-Marco R, Gomez-Ulla F, Mrejen S, Freund KB. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol. 2014;3(4):111-115.
25. Lehmann M, Bousquet E, Beydoun T, et al. Pachychoroid: an inherited condition? Retina. 2015;35(1):10-16.
35. Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244-249.
36. Horniker E. Su di unaforma di retinite central di originevasoneurotica. Ann Ottalmol. 1927;55 : 830-840.
37. Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986;84 : 799-845.
45. de Jong EK, Breukink MB, Schellevis RL, et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology. 2015;122(3):562-570.
46. Schellevis RL, Van Dijk EHC, Breukink MB, et al. Role of the complement system in chronic central serous chorioretinopathy: a genome-wide association study. JAMA Ophthalmol. 2018;136(10):1128-1136.
47. Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010;149(3):361-363.
48. Schachat APW, C.P.; Hinton, D.R. Ryan’s Retina. Volume II, 6th edition edition: Elsevier, 2018. 2976. ISBN: 978-0-323-40197-5.
56. Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986;9(1):37-41.
57. Tomasso L, Benatti L, Rabiolo A, et al. Retinal vessels functionality in eyes with central serous chorioretinopathy. Br J Ophthalmol. 2018;102(2):210-214.
59. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye Lond Engl. 2019;33(1):14-33. doi: 10.1038/s41433-018-0158-4
60. Negi A, Marmor MF. Mechanisms of subretinal fluid resorption in the cat eye. Invest Ophthalmol Vis Sci. 1986;27(11):1560-1563.
61. Wang C, Cao G-F, Jiang Q, Yao J. TNF- promotes human retinal pigment epithelial (RPE) cell migration by inducing matrix metallopeptidase 9 (MMP-9) expression through activation of Akt/mTORC1 signaling. Biochem Biophys Res Commun. 2012;425(1):33-38.
66. Carvalho-Recchia CA, Yannuzzi LA, Negrao S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834-1837.
71. Gass JD, Slamovits TL, Fuller DG, Gieser RG, Lean JS. Posterior chorioretinopathy and retinal detachment after organ transplantation. Arch Ophthalmol. 1992;110(12):1717-1722.
72. Hadcock JR, Malbon CC. Regulation of beta-adrenergic receptors by "permissive" hormones: glucocorticoids increase steady-state levels of receptor mRNA. Proc Natl Acad Sci USA. 1988;85(22):8415-8419.
76. Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrão S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23(1):1-7
77. Piccolino FC, Eandi CM, Ventre L, Rigault De La Longrais, RC. Grignolo, FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003;23(6):752-763.
78. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139(1):87-99.
79. Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48 : 82-118.
80. Chhablani J. Central serous chorioretinopathy. Elsevier Science, 2019.27-34. ISBN: 9780128168004.
82. Aggio FB, Roisman L, Melo GB, Lavinsky D, Cardillo JA, Farah ME. Clinical factors related to visual outcome in central serous chorioretinopathy. Retina. 2010;30(7):1128-1134.
84. Yannuzzi LA, Slakter JS, Kaufman SR, Gupta K. Laser treatment of diffuse retinal pigment epitheliopathy. Eur J Ophthalmol. 1992;2(3):103-114.
86. Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM. Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol. 2015;160(3):581-587 e1.
87. Bousquet E, Bonnin S, Mrejen S, Krivosic V, Tadayoni R, Gaudric A. Optical Coherence Tomography Angiography of Flat Irregular Pigment Epithelium Detachment in Chronic Central Serous Chorioretinopathy. Retina. 2018;38(3):629-638.
88. Bonini Filho MA, de Carlo TE, Ferrara D, et al. Association of Choroidal Neovascularization and Central Serous Chorioretinopathy With Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015;133(8):899-906.
89. Costanzo E, Cohen SY, Miere A, et al. Optical Coherence Tomography Angiography in Central Serous Chorioretinopathy. J Ophthalmol. 2015;2015 : 134783.
90. Balaratnasingam C, Freund KB, Tan AM, Sarraf D, Jampol LM, Yannuzzi LA. Bullous variant of central serous chorioretinopathy: expansion od phenotypic features using multimethod imaging. Ophthalmology. 2016;123(7):1541-1552.
91. Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91(12):1554-1572.
95. Stanga PE, Lim JH, Hamilton P. Indocyanine green angiography in chorioretinal diseases: an evidenced-based update.Ophthalmology. 2003;110(1):15-21.
96. Yannuzzi LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol. 2011;151(1):745751. e1.
97. Iida T, Hagimura N, Sato T, Kishi S. Evaluation of central serous chorioretinopathy with optical coherence tomography. Am J Ophthalmol. 2000;129(1):16-20.
100. Shin YU, Lee BR. Retro-mode imaging for retinal pigment epithelium alterations in central serous chorioretinopathy. Am J Ophthalmol. 2012;154(1):155-163.
101. Kon Y, Iida T, Maruko I, Saito M. The optical coherence tomography-ophthalmoscope for examination of central serous chorioretinopathy with precipitates. Retina. 2008;28(6):864-869.
102. Yalcinbayir O, Gelisken O, Akova-Budak B, et al. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014;34(4):705-712.
107. Jirarattanasopa P, Ooto S, Tsujikawa A, Hirata M, Matsumoto A, Yoshimura N. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012;119(8):1666-1678.
108. Teussink MM, Breukink MB, van Grinsven MJ, Hoyng CB, Klevering BJ. OCT angiography compared to fluorescein and indocyanine green angiography in chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2015;56 : 5229-5237.
109. Chan SY, Wang Q, Wei WB, Jonas, JB. Optical Coherence Tomographic Angiography in Central Serous Chorioretinopathy. Retina. 2016;36(11):2051-2058.
110. Rabiolo A, Zucchiatti I, Marchese A, et al. Multimodal retinal imaging in central serous chorioretinopathy treated with oral eplerenone or photodynamic therapy. Eye (Lond). 2018;32(1):55-66.
111. Pichi F, Morara M, Veronese C, Ciardella AP. The overlapping spectrum of flat irregular pigment epithelial detachment investigated by optical coherence tomography angiography. Int Ophthalmol. 2018;38(3):975-983.
115. Holz FG, Bindewald-Wittich A, Fleckenstein M, Scholl HPN, Steffen Schmitz-ValckenbergFam-Study Group. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463-472.
116. Maruko I, Iida T, Ojima A, Sekiryu T. Subretinal dot-like precipitates and yellow materiál in central serous serous chorioretinopathy. Retina. 2011;31(4):759-765.
117. Ehrlich R, Mawer NP, Mody CH, Brand CS, Squirrell D. Visual function following photodynamic therapy for central serous chorioretinopathy: a comparison of automated macular microperimetry versus best-corrected visual acuity. Clin Exp Ophthalmol. 2012;40(1):e32-39.
120. Lai TY, Lai RY, Ngai JW, Chan WM, Li H, Lam DSC. First and second-order kernel multifocal electroretinography abnormalities in acute central serous chorioretinopathy. Doc Ophthalmol. 2008;116(1):29-40.
121. Caccavale A, Romanazzi F, Imparato M, et al. Central serous chorioretinopathy: a pathogenetic model. Clin Ophthalmol. 2011;5 : 239-243.
123. Chrapek O, Rehak J. Léčba centrální serózní chorioretiniopatie-naše zkušenosti. [Treatment of central serous chorioretinopathy-personal experience]. Cesk Slov Oftalmol. 2002;58(1):51-56. Czech.
124. Alkin Z, Osmanbasoglu OA, Ozkaya A, Karatas G, Yazici AT, Demirok A. Topical nepafenac in treatment of acute central serous chorioretinopathy. Med Hypothesis Discov Innov Ophthalmol. 2013;2(4):96-101.
125. Schwartz R, Habot-Wilner Z, Martinez MR, et al. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol. 2017;95(7):e610-e618.
126. Rahimy E, Pitcher JD, 3rd, Hsu J, et al. A Randomized Double-Blind Placebo-Control Pilot Study of Eplerenone for the Treatment of Central Serous Chorioretinopathy (Ecselsior). Retina. 2018;38(5):962-969.
127. Cakir B, Fischer F, Ehlken C, et al. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2151-2157.
128. Boscia F. When to treat and when not to treat patient with central serous retinopathy. Retina Today. [online], April 2010. Available from: https://retinatoday.com/articles/2010-apr/when-to-treat-and-not-to-treat-patients-with-central-serous-retinopathy
129. Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophhalmol. 1984;68(11):815-820.
130. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(3 Suppl):1-139.
131. Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889-905.
132. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003;23(3):288-298.
133. Koss M. Treating Chronic Central Serous Chorioretinopathy: The goal is a treatment with inceased Access and decreased effects. Retina Today. 2013;8 : 68-70.
134. Lai TY, Chan WM, Li H, Lai RYK, Liu DTL, Lam DSC. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006;90(7):869-874.
135. Fujita K, Imamura Y, Shinoda K, Hashizume K, Mizota A, Yuzawa M. One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology. 2015;122(3):555-561.
136. Tseng CC, Chen SN. Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br J Ophthalmol. 2015;99(8):1070-1077.
137. Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45(3):195-214.
138. Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, Viestenz A, Naumann GOH. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002;120(6):835-844.
139. Maier M, Valet V, Feucht N, Lohmann CP. Therapieoption bei chronischer Chorioretinopathia centralis serosa [Therapy options for chronic central serous chorioretinopathy]. Ophthalmologe. 2011;108(11):1027-1031. German.
140. Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003;110(1):15-21; quiz 22-3.
141. Nicolo M, Eandi CM, Alovisi C, et al. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2014;157(5):1033-1037.
142. van Dijk EHC, Fauser S, Breukink MB, Downes SM, Hoyng CB, Boon CJF. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology. 2018;125(10):1547-1555.
143. Manethová K, Ernest J, Hrevuš M, Jirásková N. Assessment of the Efficacy of Photodynamic Therapy in Patients with Chronic Central Serous Chorioretinopathy. Cesk Slov Oftalmol. 2019;75(6):298-308. doi:10.31348/2019/6/2
144. Bae SH, Heo J, Kim C, Park TK, Moon SW, Chung H. Low-fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one-year results of a randomized trial. Ophthalmology. 2014;121(2):558-565.
145. Chan WM, Lai TY, Liu DT, Lam DSC. Intravitreal bevacizumab (avastin) for choroidal neovascularization secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy, or of idiopathic origin. Am J Ophthalmol. 2007;143(6):977-983.
146. Lim JW, Kim MU, Shin MC. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina. 2010;30(9):1465-1471.
147. Myslík Manethová K, Ernest J. Retrospective analysis of the presence of choroidal neovascularisation using optical coherence tomography angiography in the treatment of chronic central serous chorioretinopathy with the aid of photodynamic therapy. Cesk Slov Oftalmol. 2021;77(3):122-131. doi: 10.31348/2021/14
148. Konstantinidis L, Mantel I, Zografos L, Ambresin A. Intravitreal ranibizumab in the treatment of choroidal neovascularization associated with idiopathic central serous chorioretinopathy. Eur J Ophthalmol. 2010;20(5):955-958.
149. Broadhead GK, Chang A. Intravitreal aflibercept for choroidal neovascularisation complicating chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):979-981.
150. Asahi MG, Chon AT, Gallemore E, Gallemore RP. Photodynamic therapy combined with antivascular endothelial growth factor treatment for recalcitrant chronic central serous chorioretinopathy. Clin Ophthalmol. 2017;11 : 2051-2056.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2023 Issue Ahead of Print-
All articles in this issue
- CURRENT VIEW OF THE SPECTRUM OF PACHYCHOROID DISEASES. A REVIEW
- ULTRASOUND EXAMINATION OF THE ORBIT IN PATIENTS WITH THYROIDASSOCIATED ORBITOPATHY – EXAMINATION GUIDE AND RECOMMENDATIONS FOR EVERYDAY PRACTICE. A REVIEW
- COMPUTER TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING OF THE ORBIT IN THE DIAGNOSIS AND TREATMENT OF THYROID-ASSOCIATED ORBITOPATHY – EXPERIENCE FROM PRACTICE. A REVIEW
- DETERMINATION OF CORNEAL POWER AFTER REFRACTIVE SURGERY WITH EXCIMER LASER: A CONCISE REVIEW
- Eyelid Schwannoma. A Case Report
- The Current State of Artificial Intelligence in Neuro-Ophthalmology. A Review
- Central Serous Chorioretinopathy. A Review
- Therapy for Vitreous Seeding Caused by Retinoblastoma. A Review
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Central Serous Chorioretinopathy. A Review
- ULTRASOUND EXAMINATION OF THE ORBIT IN PATIENTS WITH THYROIDASSOCIATED ORBITOPATHY – EXAMINATION GUIDE AND RECOMMENDATIONS FOR EVERYDAY PRACTICE. A REVIEW
- CURRENT VIEW OF THE SPECTRUM OF PACHYCHOROID DISEASES. A REVIEW
- COMPUTER TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING OF THE ORBIT IN THE DIAGNOSIS AND TREATMENT OF THYROID-ASSOCIATED ORBITOPATHY – EXPERIENCE FROM PRACTICE. A REVIEW
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

