-
Medical journals
- Career
Prevalence and the role of CCR5Δ32 heterozygosity in disease progression in HIV positive patients in the Czech Republic
Authors: L. Sácká 1; J. Hodek 1; L. Machala 2; M. Malý 3; J. Weber 1
Authors‘ workplace: Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Prague, Czech Republic 1; Department of Infectious Diseases, Third Faculty of Medicine, Charles University and Hospital Na Bulovce, Prague, Czech Republic 2; National Institute of Public Health, Prague, Czech Republic 3
Published in: Epidemiol. Mikrobiol. Imunol. 68, 2019, č. 3, s. 138-143
Category: Original Papers
Overview
Background: Entry of human immunodeficiency virus type 1 (HIV-1) in target cells is enabled by CD4 receptor and one of two co-receptors, CXCR4 or CCR5. Deletion of 32 bp in CCR5 gene (CCR5Δ32) in both alleles provides strong but not absolute resistance to HIV-1 infection and deletion in one allele slows disease progression to AIDS. Here, we analyzed the prevalence and the role of CCR5Δ32 heterozygosity on the disease progression in HIV positive patients in the Czech Republic.
Patients and methods: A total of 92 HIV-1 seropositive subjects that included 80 Czech individuals from the AIDS center in the Hospital Na Bulovce in Prague were enrolled in CCR5 genotyping as a part of a study of the role of HIV fitness on disease progression. DNA was extracted from patient’s peripheral blood mononuclear cells and subjected to real-time PCR with specific probes detecting wild-type and 32 bp-deleted CCR5 variants. A subgroup of 74 antiretroviral therapy-naive patients with more than one year of follow-up was used to determine the role of the CCR5Δ32 heterozygous phenotype in disease progression.
Results: CCR5Δ32 was found heterozygous in 23.8% of 80 Czech HIV-1 seropositive individuals which is very similar to 21% and 24% prevalence reported in HIV negative Czech population. Homozygous mutant variant was not detected. In CCR5Δ32 heterozygous group we observed slightly higher mean CD4+ T-cell count and lower mean plasma viremia levels.
Conclusions: Overall, our study indicates no obvious benefit of CCR5Δ32 heterozygosity on HIV transmission and only small benefit on disease progression in the Czech HIV-1 cohort.
Keywords:
HIV-1 – CCR5 co-receptor – CCR5Δ32 – heterozygous polymorphism – disease progression
INTRODUCTION
Human immunodeficiency virus (HIV) entry into target cells is facilitated both by CD4 receptor and co-receptors. The CXCR4 and CCR5, members of the seven-transmembrane G protein-coupled receptor family, serve as the major co-receptors for T-cell line-tropic and macrophage-tropic HIV-1 strains, respectively (reviewed in [1, 2]). Soon after their discovery [3–5] it was shown that persons heterozygous for a 32 base pair deletion in gene encoding CCR5 (CCR5Δ32) have slower progress to AIDS than individuals with two wild type alleles [6–11]. Moreover, individuals homozygous for CCR5Δ32 showed high degree of resistance to HIV-1 infection but not absolute as already several cases of HIV infection in these subjects were documented ([12] and citations herein).
Population surveys showed highest prevalence of CCR5Δ32 deletion in persons of European descent, lower prevalence in people from western Asia, sporadic in individuals from northern Africa and almost no prevalence in native Africans and Asians [13–17]. In healthy unrelated Czech individuals the CCR5Δ32 was found heterozygous in 21% persons and only one individual out of 386 (0.3%) tested persons carried both mutant alleles [18]. Another study performed in the HIV-1 negative Czechs found in approximately double number of the subjects on average 24% individuals with mutant CCR5 allele [19]. To the best of our knowledge, the only report about prevalence of CCR5Δ32 deletion in HIV-1 positive persons was presented at 14th European Congress of Clinical Microbiology and Infectious Diseases in Prague in 2004. In this study homozygous mutant genotype was not found, but 18% of individuals carried one mutant CCR5 allele [20].
There is a clear benefit of homozygous deletion in CCR5 gene that results in absence of functional co-receptor on the cell surface and inability of CCR5-tropic HIV-1 to enter cells. Less evident is the advantage for HIV-1 infection and pathogenesis in the case of heterozygous deletion in CCR5. It was shown that in spite of considerable innate variability, CCR5 expression levels in peripheral blood mononuclear cells (PBMCs) are markedly reduced in CCR5-+/Δ32 heterozygous individuals compared to CCR5 wild type individuals [21]. In addition, lower infection of PBMCs from CCR5 heterozygous individuals with CCR5-tropic HIV-1 isolates was documented [22]. In contrast, another study demonstrated that overexpression of CCR5Δ32 did not markedly impair the cell surface density or function of co-expressed wild-type receptor either in human epithelial cells or Jurkat T cells [23]. Additional molecular mechanism was proposed where mutant CCRΔ32 protein is retained in endoplasmic reticulum and exert transdominant negative effect on wild-type CCR5, preventing exit from this compartment [24, 25]. Moreover, this mechanism is supported by showing that expression of recombinant CCR5Δ32 in human PBMCs provides protection against both CCR5 - and also CXCR4-tropic HIV-1 strains [26]. It demonstrates ability of truncated CCR5 protein to form heterodimeric complexes with wild type CCR5 and CXCR4 resulting in reduced cell surface expression of both co-receptors [26].
Lower CCR5 co-receptor density on the cell surface and ability of truncated CCR5 protein to scavenge functional co-receptors is the best explanation to date for protective role of CCR5Δ32 heterozygosity. CCR5 co-receptor density was correlated with viral load and immunological progression [27, 28]. Controversial is the benefit of CCR5Δ32 heterozygosity for HIV transmission. Among studies reporting that CCR5Δ32 heterozygotes are more protected from HIV-1 infection than CCR5 wild type homozygous individuals is cross-sectional and longitudinal analysis in large MSM cohort [29], analysis in small group of heterosexual discordant couples [30], analysis in Caucasian cohorts [31], analysis in Greek [32], Italian [33] and Brazilian cohorts [34]. On the contrary, no beneficial effect on HIV transmission was observed in study on heterosexual transmission in Scotland [35], in several cohorts in the USA [8], in cohort of drug users and hemophiliacs in Italy [36], in analysis in Chinese [37] and Columbian population [38]. A larger meta-analysis of 18 studies on more than 12000 subjects found no significant effect of CCR5Δ32 heterozygosity on protection from HIV-1 infection [39]. Better consensus in the HIV field we find on slightly delayed progression to AIDS in HIV-1-infected individuals carrying single CCR5Δ32 allele. Immediately after discovery of Δ32 deletion in CCR5 gene, several studies showed 2-4 years delay in progression to AIDS, long-term nonprogression of HIV disease, survival advantage, and lower all-cause mortality [6–10, 40–43]. A large meta-analysis from 15 cohorts from Western Europe, Africa, USA and Australia quantified and expressed the benefit of CCR5Δ32 mutation as 25% slower progression to AIDS and 35% slower progression to death [44]. In this paper, we analyzed the prevalence and the role of CCR5Δ32 heterozygosity on the disease progression in HIV positive patients in the Czech Republic.
MATERIALS AND METHODS
Subjects and samples
The CCR5 genotype analyses were performed as a part of a study of The Role of HIV Fitness on Disease Progression in the Absence of Antiretroviral Treatment. Between May 2012 and June 2013 92 HIV-1 seropositive subjects were enrolled in the study at AIDS center in the Hospital Na Bulovce in Prague. Subjects signed an informed consent that included provision for genetic testing such as CCR5 genotype determination (6.2.2012/6015/EK-Z). Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by gradient centrifugation on Ficoll Paque Plus (GE Healthcare Life Sciences, Chicago, Illinois, USA) and stored at -80 °C.
DNA extraction and real time PCR amplification
DNA was extracted from thawed PBMCs using QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany), eluted in 100 µl of deionized water and used as a template for real-time PCR. Primers and probes were designed based on the published CCR5 sequence (GenBank accession number NG_012637) and were as follows: CCR5 forward 5’-GGGTGGTGACAAGTGTGAT-3’, CCR5 reverse 5’-CAGATGACCATGACAAGCA-3’, probe detecting wild-type CCR5 variant ROX-CAGTCAGTATCAATTCTGGAAGAATTTC-BHQ (wild-type probe) and probe detecting 32 bp-deleted variant FAM-CTCATTTTCCATACATTAAAGATAGTCATC-BHQ (Δ32 probe). PCR was performed in 20 µl volume with 5 mM MgCl2, 200 mM dNTPs (both Thermo Fisher Scientific, Waltham, Massachusetts, USA), 0.3 μM forward primer, 0.3 μM reverse primer, 0.08 μM wt probe, 0.08 μM Δ32 probe, and 1.25 U Platinum DNA Taq polymerase (Thermo Fisher Scientific). PCR conditions consisted of one denaturation cycle (95 °C, 10 min), followed by 40 cycles of amplification (95 °C, 15 s and 60 °C, 1 min). Positive signal in FAM channel determined homozygous mutant CCR5-Δ32/Δ32 phenotype, positive signal in ROX channel determined homozygous wild type phenotype, and positive signal in both ROX and FAM channels determined heterozygous CCR5-+/Δ32 phenotype. Resolution on 2% agarose gel confirmed 206 bp long PCR product for wild type CCR5 allele and 174 bp long PCR product for Δ32 allele.
Statistical analysis
CD4+ T lymphocyte counts were determined during routine patient visits in the Hospital Na Bulovce and viral loads were measured at the National Institute of Public Health in Prague. CD4+ T-cells and logarithms of HIV-1 viral load results are expressed as mean values and standard deviations. Differences in CD4+ T-cell slopes distribution between groups were analyzed using the Mann-Whitney test. Slopes were determined for each patient separately from all CD4+ T-cell values during the follow-up period up to the initiation of antiretroviral treatment. Mean CD4+ T-cell slopes are expressed as mean values and standard errors of the mean. All analysis and graphs were performed using GraphPad Prism v.8.0.2 (GraphPad Software, La Jolla, USA).
RESULTS
In the cohort of 92 HIV positive patients of Caucasian decent we detected 23.9% of heterozygous CCR5Δ32 genotype and no homozygous CCR5Δ32 genotype (Table 1). The removal of six Slovaks, two Ukrainians, one Slovenian, one Belorussian, one Armenian, and one Hungarian from the analysis led to almost identical percentage of heterozygous CCR5Δ32 genotype (23.8%) in the remaining 80 Czech patients. In our dataset there were only five women (one CCR5Δ32 heterozygous and four CCR5 wild-type homozygous), which reflects the trend in the Czech Republic as almost 90% of new infections are in men.
1. Percentages of each CCR5 genotype among HIVseropositive individuals in study population 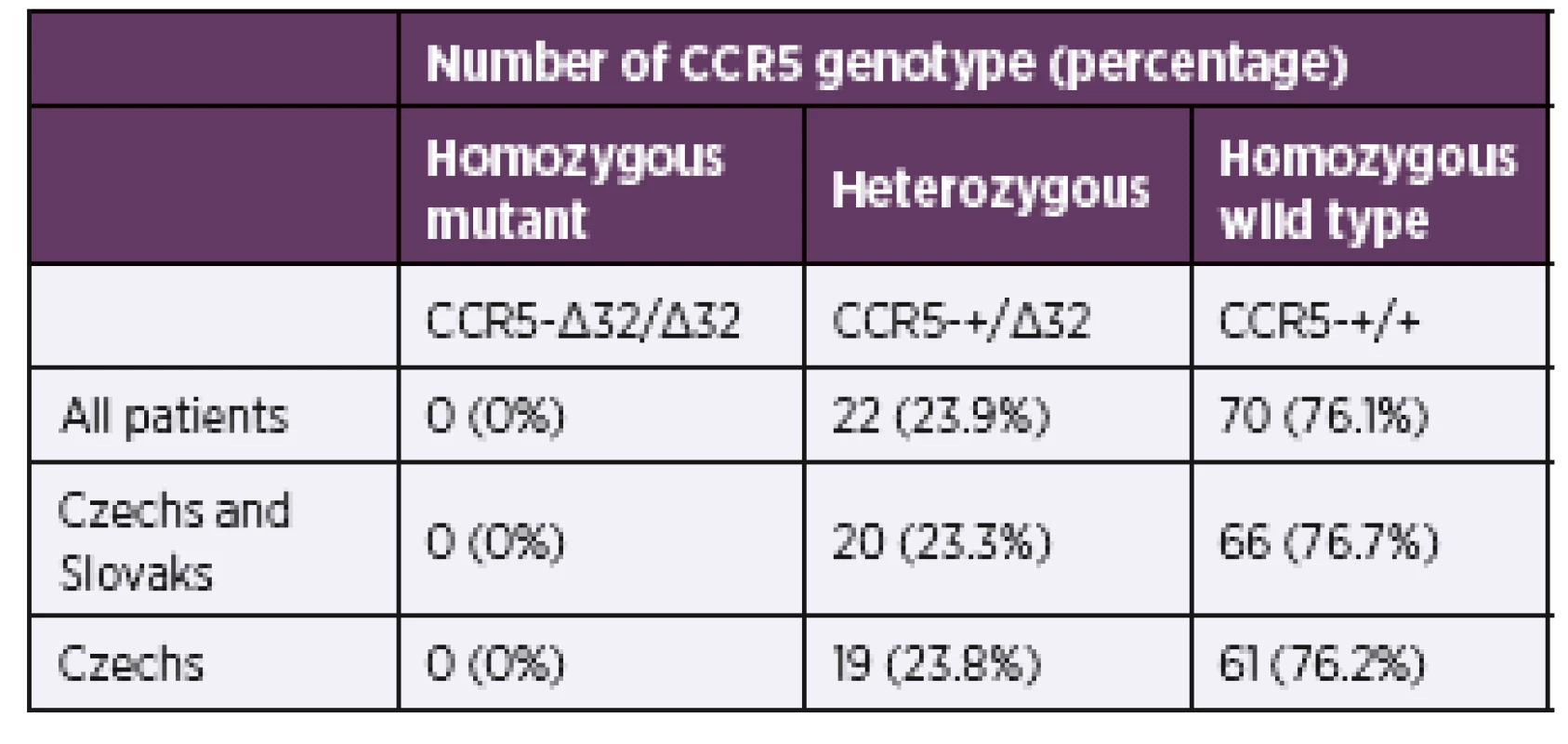
To investigate the role of the CCR5Δ32 heterozygous phenotype in disease progression we selected a subgroup of antiretroviral therapy-naive patients with more than one year of follow-up (Table 2). This led to selection of 74 patients (71 men and three women) with the same distribution of Δ32 heterozygosity. Both groups, CCR5Δ32 heterozygotes and wild type homozygotes, had similar age range and follow-up range. There was slightly higher mean CD4+ T-cell count (p = 0.055) and lower mean plasma viremia levels (p = 0.023) in heterozygotes. It is worth noting that the mean CD4+ T-cell count decline was slightly lower in wild type homozygotes but this reduction did not reach statistical significance (p = 0.78). The trend was changing with increased follow-up. Consequently, analysis of patients with four and more years (total 35 patients) showed significantly lower decline of CD4+ T-cell that led to loss of two cells each month more in wild-type persons than in heterozygous persons (p = 0.048). The overall trend is graphically evident by the small shift to the right in the distribution of slopes of CD4+ T-cell change for CCR5Δ32 heterozygotes (Figure 1a). Similarly, the small reduction in mean plasma viremia level of 0.35 log RNA copies/ml in heterozygotes is better evident by the shift to the left in the distribution of viral loads for patients carrying Δ32 deletion (Figure 1b).
2. Demographic, clinical and virological parameters for antiretroviral therapy-naive patients with more than 1-year of follow-up 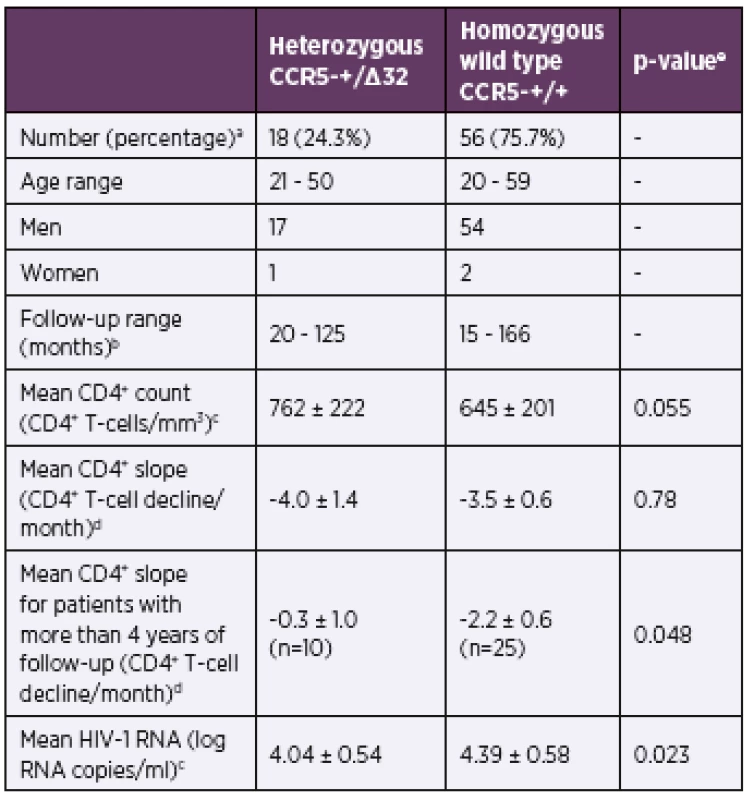
aNumber and percentage of patients for each group. bRange of months that the patients had been monitored up to the initiation of antiretroviral treatment. cMean and standard deviation of CD4+ T-cell count and HIV-1 viral load from all available values determined during the follow-up period up to the initiation of antiretroviral treatment. dMean and standard error of the mean of CD4+ T-cell count decline (slope) determined as average of individual patient’s CD4+ T-cell slopes using all CD4+ count values determined during the follow-up period up to the initiation of antiretroviral treatment. eMann-Whitney test, n number of patients. 1. The CCR5 genotype and disease progression for antiretroviral therapy-naive patients with more than 1-year of follow-up The probability distributions of the CD4+ T-cell change per month (a) and plasma viremia levels (b) are depicted for the wild-type homozygotes (n = 56; grey bars) and CCR5Δ32 heterozygotes (n = 18; black bars). n – number of patients. 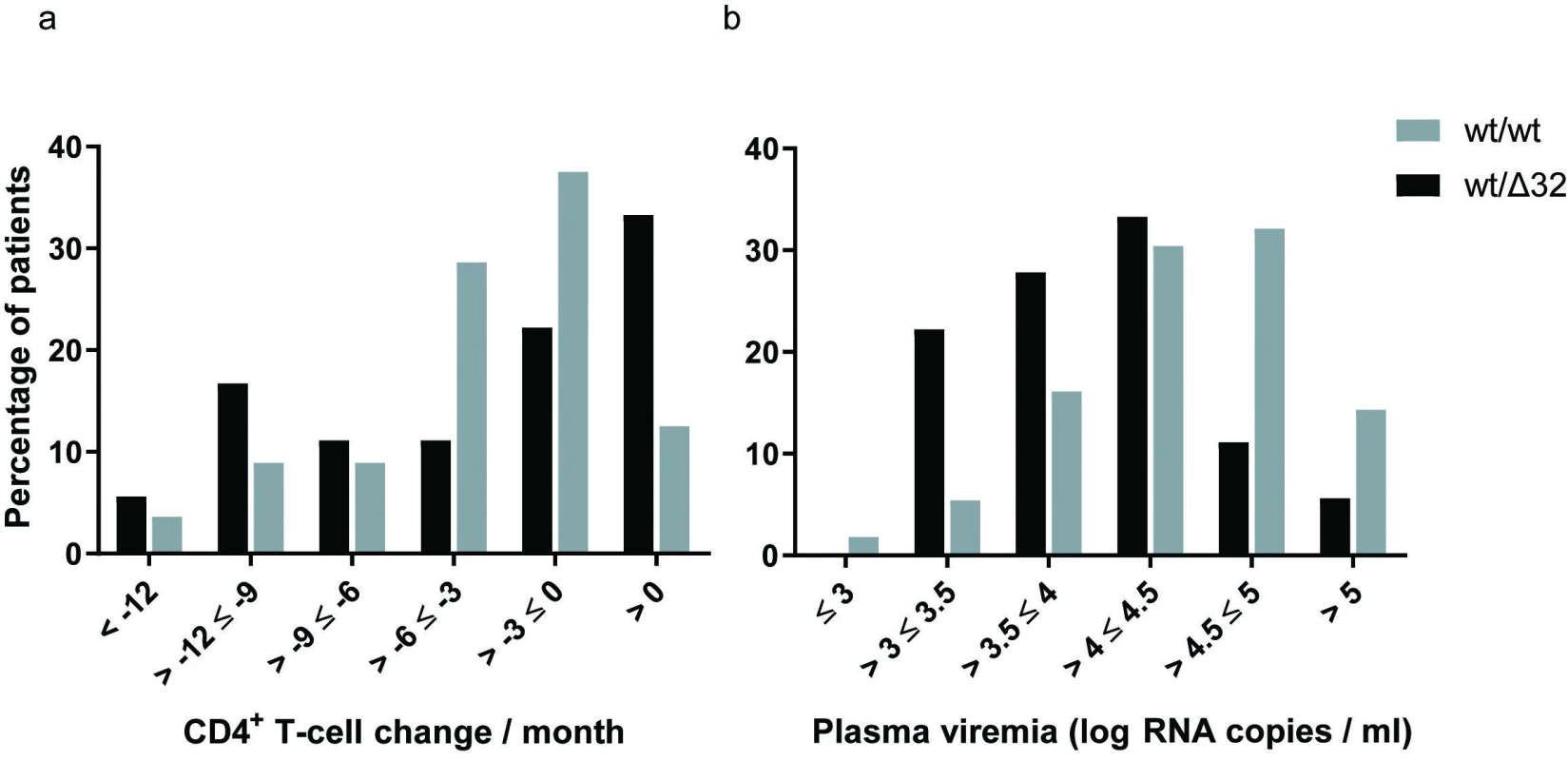
DISCUSSION
The only work presented on CCR5Δ32 allele in HIV-1 positive persons in the Czech Republic showed about 5% lower occurrence of CCR5 32-bp heterozygous deletion and with agreement with our study no homozygous mutant genotype was found [20]. Comparing the prevalence of CCR5Δ32 heterozygotes in HIV positive persons in our study with 21% and 24% prevalence reported in HIV negative Czech population indicates no obvious benefit of CCR5Δ32 heterozygosity on HIV transmission in the Czech Republic [18, 19].
In Slavic populations, there is large variability in reported prevalence of CCR5Δ32 heterozygotes between HIV negative and HIV positive population. In polish population Wasik et al reported higher, but not statistically significant, prevalence of CCR5Δ32 mutant allele among seronegative persons (13.6%) compared with HIV positive persons (9.7%) [45]. The small sample set of only 59 seronegative participants could explain the difference in comparison with study from 2000 where 861 individuals were examined that showed 10.9% frequency of Δ32 and 20.3% heterozygotes [46]. Interestingly, more recent study in the Polish population showed protective effect of CCR5Δ32 heterozygous phenotype in the case of heterosexual exposure [47]. Here, the prevalence of CCR5Δ32 heterozygosity in HIV-positive individuals infected through heterosexual contact (8.2%) was much lower than in the HIV-negative individuals (21.5%) as well as in heterosexually exposed uninfected group (25%). No significant differences in genotype representation and CCR5Δ32 allele frequencies between HIV-1 infected and uninfected were found in analyses in Slovakia [48], Russia [49, 50], and Slovenia [51].
One of the limitation of our study is the lack of knowledge of the approximate time of seroconversion. It was previously shown that difference in viremia between heterozygotes and wild type individuals reached significance in individuals with known time of seroconversion but the significance was lost in subjects with prevalent HIV-1 infection [10]. Moreover, large meta-analysis of the effect of CCR5Δ32 heterozygosity on HIV-1 disease progression has shown that heterozygous patients have diminished viremia early in the course of their disease. Compared with the HIV-1 RNA level at study entry or soon after seroconversion in wild type homozygotes, the viremia for CCR5Δ32 heterozygotes was lower by 0.18 log RNA copies / ml in seroconverters and by 0.21 log RNA copies / ml in seroprevalent patients [44].
Even slightly lower viremia can translate to slower decline of CD4+ T-cells over time [52, 53] and accordingly to lower risk of development of AIDS and death. Indeed, studies in six US cohorts, cohort of Danish homosexual men, in seroconversion SEROCO cohort, and in others showed significantly slower progression to AIDS and to death in CCR5Δ32 heterozygous group [8, 9, 44, 54]. However, CCR5Δ32 heterozygosity was not clearly associated with the risk for dying after progression to AIDS [44]. Despite the fact that with the wide use of combined antiretroviral treatment (cART) there is clear evidence of delayed disease progression and decline in mortality, the determination of patient´s CCR5Δ32 status is still clinically important. A recent study showed that CCR5Δ32 enhances the long-term survival also for those patients receiving cART [55]. Additional studies are necessary especially because previous reports did not find an association between CCR5Δ32 and survival in patients on cART [56, 57].
CONCLUSION
In conclusion, we found comparable incidence of CCR5Δ32 heterozygous genotype in HIV-1 infected persons with previously reported for HIV-1-negative Czech population, suggesting that this genotype does not protect against HIV-1 transmission. In addition, we showed small benefit of CCR5Δ32 heterozygous genotype for disease progression.
Acknowledgements
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic (LK11207) and by the Czech Academy of Sciences (RVO: 61388963). We thank the study participants and the nurses at AIDS center in the Hospital Na Bulovce.
Conflict of interest
The authors declare no conflict of interest.
Do redakce došlo dne 21. 5. 2019.
původní práce
Adresa pro korespondenci:
Mgr. Jan Weber, CSc.
Ústav organické chemie a biochemie AV ČR, v.v.i.
Flemingovo nám. 2
166 10 Praha 6
e-mail: weber@uochb.cas.cz
Sources
1. Agrawal L, Alkhatib G, Agrawal L. Chemokine receptors: emerging opportunities for new anti-HIV therapies. Expert Opin Ther Targets, 2001;5(3):303–326.
2. Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol,1999;17 : 657–700.
3. Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature, 1996;381(6584):661–666.
4. Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature, 1996;381(6584):667–673.
5. Feng Y, Broder CC, Kennedy PE, et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science, 1996;272(5263):872–877.
6. Barroga CF, Raskino C, Fangon MC, et al. The CCR5Delta32 allele slows disease progression of human immunodeficiency virus-1-infected children receiving antiretroviral treatment. J Infect Dis, 2000;182(2):413–419.
7. de Roda Husman AM, Koot M, Cornelissen M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med, 1997;127(10):882–890.
8. Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science, 1996;273(5283):1856–1862.
9. Eugen-Olsen J, Iversen AK, Garred P, et al. Heterozygosity for a deletion in the CKR-5 gene leads to prolonged AIDS-free survival and slower CD4 T-cell decline in a cohort of HIV-seropositive individuals. AIDS, 1997;11(3):305–310.
10. Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med, 1996;2(11):1240–1243.
11. Ruiz-Mateos E, Tar ancon-Diez L, Alvarez-Rios AI, et al. Association of heterozygous CCR5Delta32 deletion with survival in HIV-infection: A cohort study. Antiviral Res, 2018;150 : 15–19.
12. Henrich TJ, Hanhauser E, Hu Z, et al. Viremic control and viral coreceptor usage in two HIV-1-infected persons homozygous for CCR5 Delta32. AIDS, 2015;29(8):867–876.
13. Limborska SA, Balanovsky OP, Balanovskaya EV, et al. Analysis of CCR5Delta32 geographic distribution and its correlation with some climatic and geographic factors. Hum Hered, 2002;53(1):49–54.
14. Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell, 1996;86(3):367–377.
15. Martinson JJ, Chapman NH, Rees DC, et al. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet, 1997;16(1):100–103.
16. Novembre J, Galvani AP, Slatkin M. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol, 2005;3(11):e339.
17. Su B, Sun G, Lu D, et al. Distribution of three HIV-1 resistance-conferring polymorphisms (SDF1-3’A, CCR2-641, and CCR5-delta32) in global populations. Eur J Hum Genet, 2000;8(12):975–979.
18. Drabek J, Petrek M. 32 bp deletion in CCR-5 gene and human immunodeficiency virus epidemic in the Czech Republic. Acta Virol, 1998;42(2):121–122.
19. Šerý O, Hladilová R, Šimek V, et al. Frequency of CCR5 deletion polymorphisms in the Czech Republic population and its relationship to HIV [in czech]. 11th International workshop on AIDS, drugs and us; 2002; Prague, Czech Republic. p. 18.
20. Sedlacek D. The occurrence of CCR5 del32 and CCR2B alleles in HIV-1 positive persons in the Czech Republic. 14th European Congress of Clinical Microbiology and Infectious Diseases; May 1-4, 2004; Prague, Czech Republic.
21. Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pat-tern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med, 1997;185(9):1681–1691.
22. Kim A, Pettoello-Mantovani M, Goldstein H. Decreased susceptibility of peripheral blood mononuclear cells from individuals heterozygous for a mutant CCR5 allele to HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol, 1998;19(2):145–149.
23. Venkatesan S, Petrovic A, Van Ryk DI, et al. Reduced cell surface expression of CCR5 in CCR5Delta 32 heterozygotes is mediated by gene dosage, rather than by receptor sequestration. J Biol Chem, 2002;277(3):2287–301.
24. Benkirane M, Jin DY, Chun RF, et al. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5delta32. J Biol Chem, 1997;272(49):30603–30606.
25. Chelli M, Alizon M. Determinants of the trans-dominant negative effect of truncated forms of the CCR5 chemokine receptor. J Biol Chem, 2001;276(50):46975–46982.
26. Agrawal L, Lu X, Qingwen J, et al. Role for CCR5Delta32 protein in resistance to R5, R5X4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells. J Virol, 2004;78(5):2277–2287.
27. Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS, 2001;15(13):1627–1634.
28. Reynes J, Portales P, Segondy M, et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis, 2000;181(3):927–932.
29. Marmor M, Sheppard HW, Donnell D, et al. Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J Acquir Immune Defic Syndr, 2001;27(5):472–481.
30. Hoffman TL, MacGregor RR, Burger H, et al. CCR5 genotypes in sexually active couples discordant for human immunodeficiency virus type 1 infection status. J Infect Dis, 1997;176(4):1093–1096.
31. Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature, 1996;382(6593):722–725.
32. Papa A, Papadimitriou E, Adwan G, et al. HIV-1 co-receptor CCR5 and CCR2 mutations among Greeks. FEMS Immunol Med Microbiol, 2000;28(1):87–89.
33. Trecarichi EM, Tumbarello M, de Gaetano Donati K, et al. Partial protective effect of CCR5-Delta 32 heterozygosity in a cohort of heterosexual Italian HIV-1 exposed uninfected individuals. AIDS Res Ther, 2006;3 : 22.
34. Grimaldi R, Shindo N, Acosta AX, et al. Prevalence of the CCR5Delta32 mutation in Brazilian populations and cell susceptibility to HIV-1 infection. Hum Genet, 2002;111(1):102–104.
35. Lockett SF, Alonso A, Wyld R, et al. Effect of chemokine receptor mutations on heterosexual human immunodeficiency virus transmission. J Infect Dis, 1999;180(3):614–621.
36. Zamarchi R, Indraccolo S, Minuzzo S, et al. Frequency of a mutated CCR-5 allele (delta32) among Italian healthy donors and individuals at risk of parenteral HIV infection. AIDS Res Hum Retroviruses, 1999;15(4):337–344.
37. Wang FS, Hong WG, Cao Y, et al. Population survey of CCR5 delta32, CCR5 m303, CCR2b 64I, and SDF1 3’A allele frequencies in indigenous Chinese healthy individuals, and in HIV-1-infected and HIV-1-uninfected individuals in HIV-1 risk groups. J Acquir Immune Defic Syndr, 2003;32(2):124–130.
38. Diaz FJ, Vega JA, Patino PJ, et al. Frequency of CCR5 delta-32 mutation in human immunodeficiency virus (HIV)-seropositive and HIV-exposed seronegative individuals and in general population of Medellin, Colombia. Mem Inst Oswaldo Cruz, 2000;95(2):237–242.
39. Liu S, Kong C, Wu J, et al. Effect of CCR5-Delta32 heterozygosity on HIV-1 susceptibility: a meta-analysis. PLoS One, 2012;7(4):e35020.
40. Magierowska M, Theodorou I, Debre P, et al. Combined genotypes of CCR5, CCR2, SDF1, and HLA genes can predict the long-term nonprogressor status in human immunodeficiency virus-1-infected individuals. Blood, 1999;93(3):936–941.
41. Michael NL, Louie LG, Rohrbaugh AL, et al. The role of CCR5 and CCR2 polymorphisms in HIV-1 transmission and disease progression. Nat Med, 1997;3(10):1160–1162.
42. Mulherin SA, O’Brien TR, Ioannidis JP, et al. Effects of CCR5-Delta32 and CCR2-64I alleles on HIV-1 disease progression: the protection varies with duration of infection. AIDS, 2003;17(3):377–387.
43. Rappaport J, Cho YY, Hendel H, et al. 32 bp CCR-5 gene dele-tion and resistance to fast progression in HIV-1 infected heterozygotes. Lancet, 1997;349(9056):922–923.
44. Ioannidis JP, Rosenberg PS, Goedert JJ, et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3’A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann Intern Med, 2001;135(9):782–795.
45. Wasik TJ, Smolen J, Kruszynski P, et al. Effects of CCR5-delta32, CCR2-64I and SDF-1-3’A polymorphic alleles on human immunodeficiency virus 1 (HIV-1) infection in the Polish population. Wiad Lek, 2005;58(9–10):500–507.
46. Jagodzinski PP, Lecybyl R, Ignacak M, et al. Distribution of 32 alelle of the CCR5 gene in the population of Poland. J Hum Genet, 2000;45(5):271–274.
47. Zwolinska K, Knysz B, Rybka K, et al. Protective effect of CCR5-Delta32 against HIV infection by the heterosexual mode of transmis-sion in a Polish population. AIDS Res Hum Retroviruses, 2013;29(1):54–60.
48. Takacova M, Nogova P, Habekova M, et al. Prevalence of a 32 bp deletion in the gene for human immunodeficiency virus 1 co-receptor CCR5 in Slovak population. Acta Virol, 2008;52(4):261–264.
49. Apriatin SA, Rakhmanaliev ER, Nikolaeva IA, et al. Comparison CCR5de132 mutation in the CCR5 gene frequencies in Russians, Tuvinians, and in different groups of HIV-infected individuals. Genetika, 2005;41(11):1559–1562.
50. Kazennova EV, Aarons E, Selimova LM, et al. Comparative analysis of distribution of mutant alleles of the gene coding for the CCR-5 chemokine receptor, among people in Russia, infected and not infected with HIV-1. Vopr Virusol, 1998;43(1):30–32.
51. Poljak M, Tomazic J, Seme K, et al. Prevalence of mutant CCR5 allele in Slovenian HIV-1-infected and non-infected individuals. Acta Virol, 1998;42(1):23–26.
52. Mellors JW, Rinaldo CR, Jr., Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma [published erratum appears in Science 1997 Jan 3;275(5296):14]. Science, 1996;272(5265):1167–1170.
53. O’Brien TR, Blattner WA, Waters D, et al. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA, 1996;276(2):105–110.
54. Meyer L, Magierowska M, Hubert JB, et al. Early protective effect of CCR-5 delta 32 heterozygosity on HIV-1 disease progression: relationship with viral load. The SEROCO Study Group. AIDS, 1997;11(11):F73–78.
55. Ryabov GS, Kazennova EV, Bobkova MR, et al. Prevalence of alleles associated with HIV resistance in Russia. Genet Test, 2004;8(1):73–76.
56. Brumme ZL, Henrick BM, Brumme CJ, et al. Short communication. Association of the CCR5delta32 mutation with clinical response and >5-year survival following initiation of first triple antiretroviral regimen. Antivir Ther, 2005;10(7):849–853.
57. Parczewski M, Bander D, Leszczyszyn-Pynka M, et al. Risk of all-cause mortality in HIV infected patients is associated with clinical, immunologic predictors and the CCR5 Delta32 deletion. PLoS One, 2011;6(7):e22215.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2019 Issue 3-
All articles in this issue
- A high prevalence of viral hepatitis C in a socially excluded Roma community in Brno
- Incidence and analysis of campylobacteriosis cases in the Czech Republic in 1997–2017
- Molecular study of mycobacterial strains prevalent in the Czech Republic in 2014
- Antiviral adoptive immunotherapy using antigen-specific T cells in allogeneic hematopoietic stem cell transplant recipients
- West Nile virus (lineage 2) detected for the first time in mosquitoes in Southern Bohemia: new WNV endemic area?
- Prevalence and the role of CCR5Δ32 heterozygosity in disease progression in HIV positive patients in the Czech Republic
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Incidence and analysis of campylobacteriosis cases in the Czech Republic in 1997–2017
- Prevalence and the role of CCR5Δ32 heterozygosity in disease progression in HIV positive patients in the Czech Republic
- Antiviral adoptive immunotherapy using antigen-specific T cells in allogeneic hematopoietic stem cell transplant recipients
- A high prevalence of viral hepatitis C in a socially excluded Roma community in Brno
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

