-
Medical journals
- Career
Formulation and technology development of vaginal pessaries with probiotic activity
Authors: Zhanna Polova 1; Svitlana Aleinyk 1; Aurelii Kazak 2
Authors‘ workplace: O. O. Bogomolets National Medical University, Department of Pharmacy and Industrial Technology of Drugs 1; O. O. Bogomolets National Medical University, Department of obstetrics and gynecology № 1, Kiev, Ukraine, City Clinical Hospital № 18 2
Published in: Čes. slov. Farm., 2020; 69, 90-99
Category: Original article
Overview
Lactobacilli use in treatment and prevention of the vaginal microflora disorders, such as bacterial vaginosis and vulvovaginal candidiasis, is highly promising. The objective of this study was is to develop formulation and technology of the extemporal Lactobacillus casei (L. casei) ІМВ В-7280-containing medicinal product in the form of vaginal pessaries.
The quality control parameters were defined in accordance with the State Pharmacopeia of Ukraine (2nd edition) and included appearance, uniformity of texture, uniformity of mass and disintegration test. Lactobacilli assay was determined after preparation and within the storage period. Thus, feasible formulation and technology were selected for vaginal pessaries with an expected 6-month shelf life. The results of the hereby described research will be used for technological instruction development for extemporaneous vaginal pessaries with defined probiotic activity.
Keywords:
vaginal pessaries – Lactobacilli – vaginal dysbiosis
Introduction
Bacterial vaginosis (BV) is the most common disease of the female genital tract affecting premenopausal, postmenopausal and pregnant women (prevalence from 5% to 50%)1). It is characterized by a complete change of vaginal microbiota, reduction or loss of Lactobacilli and increased numbers of anaerobes or facultative anaerobic organism (mostly Gardnerella vaginalis and Atopobium vaginae, Prevotella species, Mobiluncus species), followed by alternation of normal рН2–6). Lactobacilli are the predominant microorganisms in the healthy human vagina microenvironment4, 7, 8), among which L. crispatus, L. gasseri, L. jenesenii, L. iners, L. vaginalis та L. rhamnosus1, 9–13) strains are the most common ones.
According to the scientific knowledge, more than 200 Lactobacilli strains are defined14), however only 1–3 of them are dominant in the individual vaginal microenviroment12).
Lactobacilli are capable of vaginal microbiota homeostasis maintenance by means of auto-aggregation, production of lactic and acetic acids, H2O2, bacteriocins and biologically active substances, co-aggregation with pathogenic microorganisms, adhesion to epithelial cells and immunomodulatory effect1, 5, 11, 15).
The healthy vaginal epithelium demonstrates a resistance to the invasion of the pathogenic microorganisms. The amount of glycogen in the surface area cells is one of the key parameters reflecting the resistance ability of the vaginal epithelium. In the process of constant renovation, old cells, being destroyed in the cycle of constant renovation, release glycogen that is the substrate for normal microbiota. The amount of glycogen in the vaginal epithelium cells may differ within the time of the year and depends on the menstrual cycle phases (the highest levels are accumulated during ovulation)16).
Women of reproductive age demonstrate vaginal pH in the rage of 3.8–4.4. However, deviations are possible from 6.6 (± 0.3) to 4.2 (± 0.2) between day 2 and day 14 of the menstrual cycle.
Vaginal glycogen serves as the nutrient source for Lactobacilli and is transformed into lactic acid by fermentation. Lactic acid enables the acidic pH of vaginal flora to keep to 3.8–4.4, causing unfavourable conditions for other microorganisms’ development. Exceptions resulting in a pH increase are observed during menstruation and within 24–48 hours after the intercourse, due to the presence of nitrogen-containing bases in sperm that are potent to neutralize the vaginal natural acidity level12, 17–19).
Adhesive capacity of Lactobacilli empowers them to compete with pathogenic and opportunistic pathogenic bacteria for nutrient sources. Adherence of Lactobacillus is mediated by lipoteichoic acid. Due to physicochemical interaction between Lactobacilli and the vaginal epithelium, allowing Lactobacilli colonization, a specific biofilm is generated to cover the mucosa and vaginal epithelium. Being attached to epitheliocytes, Lactobacilli create a solid cover on the vagina walls preventing other microorganism from adhesion to epitheliocytes receptors20, 21).
Most of Lactobacilli (70–96%) can produce hydrogen peroxide (H2O2), which interacts with cervical mucus. Due to this interaction, growth suppression and abortion of obligate anaerobes and multiplication of opportunistic pathogenic microorganisms are possible1, 12, 18, 19).
Data available show that Lactobacilli can produce antimicrobial substances such as bacteriocins (nisin, diplocin, lactospretcin, helveticin, calycin, microcin, pesticin, pyocin, etc.). These are low-molecular-weight peptides capable of adjusting to specific microbial cell receptors in order to cause membrane destabilization and endoplasma leaks resulting in the destruction of pathogenic, opportunistic pathogenic microorganisms and fungi16). Lactocidin, acidolin and lactacin B effects are significant for maintaining healthy vaginal microbiome. Lactocins B, F, J, M, acidolin and lactocidin, bulgaricin, lactobrevin, helveticin, lactolin, and reuterin demonstrate an ability to inhibit growth and multiplying of a vast number of bacilli, Clostridia, Saccharomycetes, Streptococcus, Staphylococcus, Enterobacteriaceae, Pseudomonas, Listeria, and Candida22, 23).
Lactobacilli protective function is realized by means of various biologically active substances such as glycolipids, lipopeptides, polysaccharide-peptide complexes, phospholipids, fatty acids and neutral lipids that contribute to pathogens’ growth suppression and prevent microorganisms’ adhesion to epithelial cells19).
The Lactobacilli immunostimulatory effect is revealed through activation of macrophages, accumulation of phagocytes, cytokine synthesis and an Ig level increase11, 15).
Data obtained from literature review have shown numerous studies on efficacy and wide prospective of various Lactobacilli strains use in various vaginal dosage forms prophylaxis and treatment of vaginal flora disorders (Table 1).
1. Several studies with different dosage forms for vaginal use containing Lactobacillus 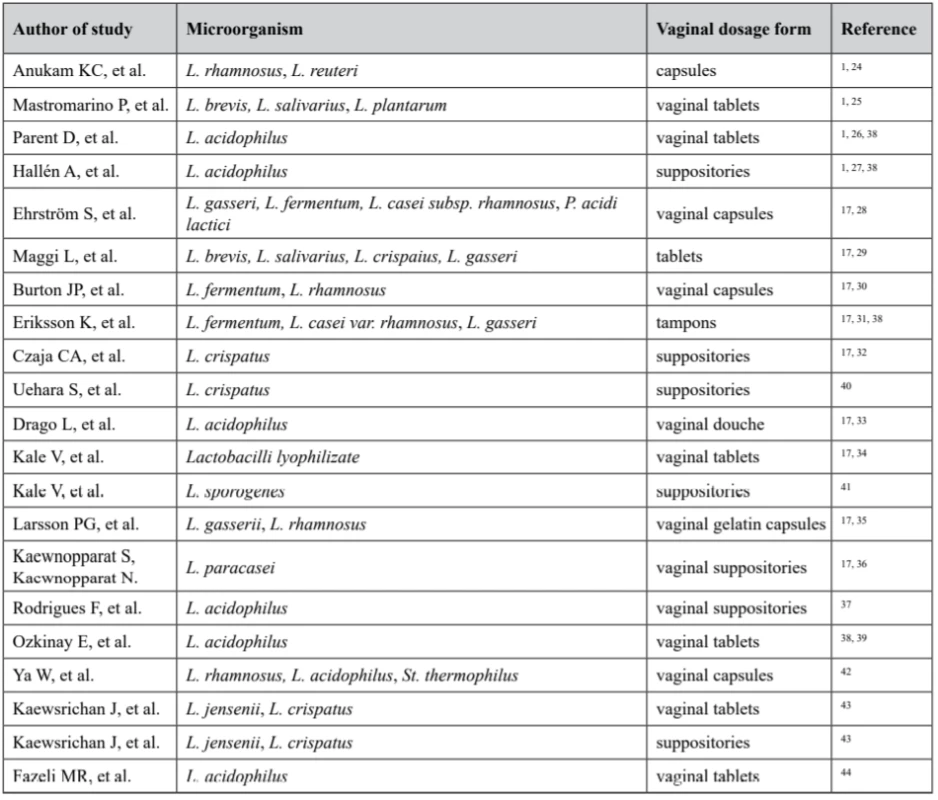
Thus, vaginal dosage forms available around the world include creams, gels, tablets, capsules, pessaries, foams, ointments, films, tampons, rings, and douches17). Numerous researches demonstrate profound use of Lactobacilli-containing dosage forms for vaginal delivery.
The effect of vaginal application of medicinal products is achieved by uniform distribution throughout the vaginal cavity. The choice of dosage form depends on the expected therapeutic effect. Semi-solid and solid dosage forms are required for local effect, achieved mostly by pessaries and vaginal tablets widely used in gynaecological practice45, 46).
Extemporaneous production is an integral part of the pharmaceutical market in highly developed European countries. In Europe two types of pharmaceutical preparations are prepared in drugstores: extemporaneous preparations and stock preparations47).
Pharmaceutical compounding in the European Union is carried out in accordance with the Good Pharmacy Practice standards. In Ukraine, this pharmacy production activity is also regulated by appropriate orders, instructions and other legal acts.
The objective of this study is the formulation and technology development of vaginal pessaries with the substance L. casei for the treatment and prevention of vaginal flora disorders (extemporaneous production), as well as the quality control of the dosage form according to the requirements of the State Pharmacopoeia of Ukraine (2nd edition).
Experimental part
Materials
Freeze-dried L. casei ІМВ В-7280 (1013 CFU/g) extracted from biological material and synthesized in the laboratory at Danylo Zabolotny Institute of Microbiology and Virology NASU were used as the active substance of vaginal pessaries48, 49). Samples were prepared with an approximate count of Lactobacilli 109 CFU per 1 pessary.
As the base for vaginal pessaries, the following components were used: hard fat Novata®B РH with the melting point of 35.2 °C – Ph. Eur. (BASF, Germany), polyoxyethylene glycols (PEGs) with the molecular weight of 1500 and/or 4000 (JSC Fine Organic Synthesis Plant Barva, Ukraine), PEG Kollisolv® 400 (BASF, Germany). Polysorbate 80 (ERCA, Italy) was used as the emulsifier and purified water as the solvent.
Preparation of vaginal pessaries
Pessaries were compounded at the scientific laboratory of O. O. Bogomolets National Medical University Pharmacy and Industrial Technology of Drugs Department. For pessaries preparation, diphilic base was chosen in order to avoid main disadvantages of both hydrophilic and lipophilic bases. Moulding was used as the pessaries preparation method. The composition of the pessaries samples is presented in Table 2.
2. Composition of the experimental samples 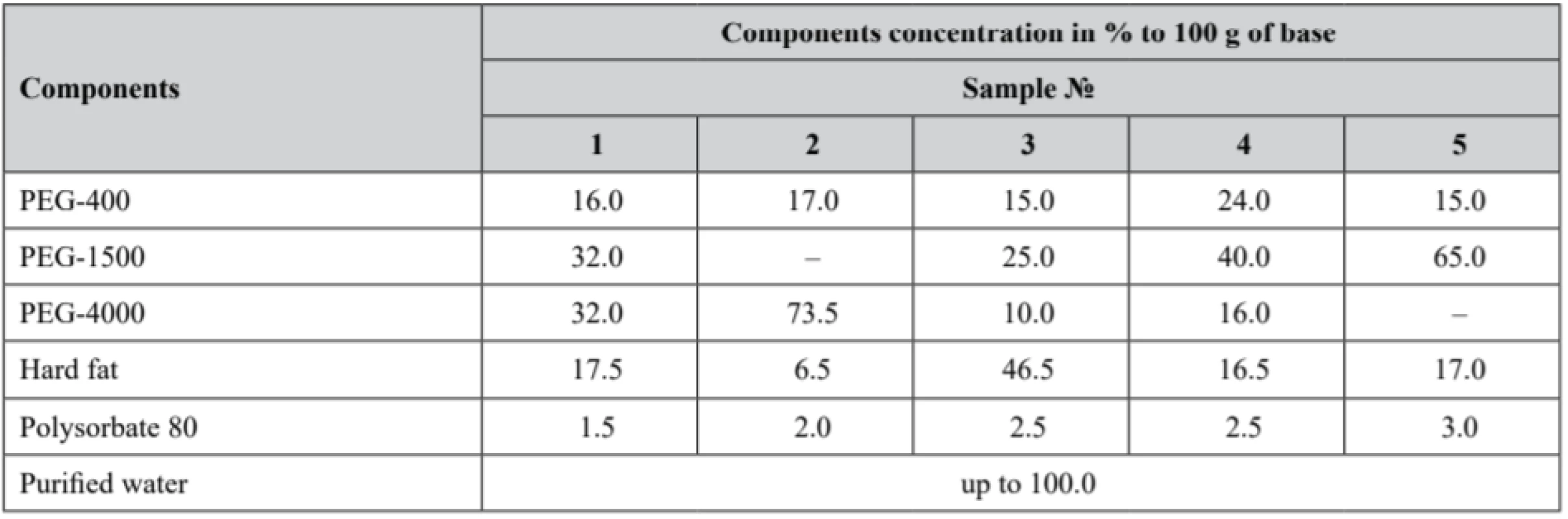
Technology of preparation
Hydrophobic and hydrophilic bases were melted separately in porcelain dishes on a VB-8 stainless steel water bath (Medika, Ukraine) at the temperature of 50–55 °С. The freeze-dried substance with probiotic activity diluted with a small (as small as possible) volume of purified water was incorporated into a base cooled down to the temperature of 40–45 °С being thoroughly stirred till uniform (homogeneous). Hydrophobic and hydrophilic fractions were mixed with the emulsifier polysorbate 80 and poured into a fluoroplastic (teflon) suppository mould F-4 (TC 6-05-810-88) (ТМ Promvit, Ukraine) (claimed mass of 1 pessary made in the mould matrix was 3.9 g), firstly cooled down for 1 hour at room temperature, then refrigerated at the temperature of 2–8 °С until the pessaries were fully congealed.
Methods
Microbiological study
The number of Lactobacilli was determined in the prepared sample by a microbiological method using pessaries prior melted in a thermostat (within 1 hour at the temperature of 37 °С). A fixed volume of the melted pessaries was applied onto a Petri dish covered with solid selective growth media. The nutrient medium MRSA (Merck, Germany) was used to identify L. casei IMB B-7280 strain in pessaries.
The following growth media were used for opportunistic pathogenic microorganisms’ microbiological tests; BAIRD-PARKER-Agar (Merck, Germany) was utilized as the selective medium for Staphylococcus; KF - -Streptococcusagar (Merck, Germany) was used as the selective medium for Streptococcus; ENDO (Farmactive Ltd., Ukraine) was chosen as the selective medium for coliform bacteria; Saburo (Farmactive Ltd., Ukraine) served as the selective medium for microscopic fungi. The bacteria count was counted up in a Petri dish after cultivation within 24 hours at 37 °C, presuming that one colony equals one bacterium.
Samples of pessaries as a particular dosage form were tested for quality parameters (appearance, uniformity of texture, uniformity of mass, disintegration) defined in accordance with the State Pharmacopeia of Ukraine (2nd edition).
Appearance and uniformity of texture tests
Pessaries must be of the same size and shape. Uniformity of texture is defined by splitting the pessary longitudinally. No inclusions on the split are acceptable, while axial air cavities inside or funnel-shaped indentation are allowed.
Uniformity of mass: 20 sample units must be taken at random, each weighed separately with a following average mass calculation. The individual deviation must be not more than ± 5%.
Disintegration (method 2.9.2): hydrophilic-based pessaries are examined in 60 minutes, while the hydrophobic-based ones undergo examination in 30 minutes. The aim of disintegration test is to determine whether a pessary sample softens or disintegrates within the required time period. Disintegration tests were performed at a suppository and pessaries disintegration tester PTS 3Е (PHARMA TEST, Austria). Three pessaries of each prepared samples were placed into a perforated basket, then dumped into a water bath and heated up to 37 °С, being turned every 10 minutes through 180 °. The procedure identified the time needed for samples disintegration, leading to the following effect: melted fatty components piled on the surface, while soluble components were completely dissolved.
Stability test
All five samples of vaginal pessaries were kept in glass containers at room temperature (25 ± 2 °C) and 2–8 °C for 6 months. At appropriate time intervals 0, 1 month, 3 months and 6 months, the assay of Lactobacilli was determined by a microbiological method using MRSA (Merck, Germany) as the medium.
Results and discussion
Diphilic base was chosen for vaginal pessaries formulation based on the peculiarity of its properties.
The main advantages of lipophilic bases include an optimal melting point range (30–36 °C), good release of water-soluble drugs, and no irritability on the mucous membranes. Compared to theobroma oil, the positive properties of semisynthetic hard fat bases can be seen in the next points: solidifying points unaffected by overheating, good resistance to oxidation because of a lower content of unsaturated fatty acids and good water-absorbing capacities. Moreover, no mould lubricant is necessary to use because of excellent volume contractility on cooling. On the other hand, hard fat also shows some disadvantages: brittleness if cooled rapidly, impossibility to use a refrigerator during preparation, and low viscosity in melted state. The latter factor could be responsible for drug particles sedimentation during preparation and non-uniform drug distribution causing local irritability50–52).
Hydrophilic pessaries need water for the medicine to be successfully dissolved and absorbed. Unlike hydrophobic-based pessaries, hydrophilic ones can dissolve in physiological fluids50) In accordance with the accepted general assumption, PEGs have long been claimed to be favourable compounds of the hydrophilic base due to their chemical stability, inertia, and wide availability. Basic quality control parameters of pessaries such as mechanical hardness, solubility, and appropriate softening temperature can be easily achieved by using PEGs with different molecular weights51). However, PEGs have significant disadvantages compared to fats. Firstly, they tend to be more chemically reactive, therefore the preparation technology must be carefully followed to avoid organoleptic parameters of pessaries being non-compliant with the norms set. Secondly, the rate of water-soluble substances release decreases noticeably with the PEG molecular weight increase. In addition, PEGs appear to be more irritating to mucous membranes37, 51).
Thus, the diphilic base was considered to be a highly promising option to avoid both hydrophobic and hydrophilic bases demerits and was selected for Lactobacilli incorporation. Lactobacilli substance is in fact a water-soluble lyophilizate, consequently, a better release rate is expected from hydrophilic bases, since they allow direct diffusion on substances into physiological fluids. Still, the use of a PEGs mixture only is regarded to be non-feasible, since these ingredients, being highly hydrophilic and hygroscopic, may cause mucous dehydration and thus, lead to a substances absorption rate decrease52).
The first phase of the research was devoted to a microbiological study of pessaries to identify an assay of Lactobacilli (Table 3) in the experimental samples that also were tested for microbiological purity (Table 4).
3. Lactobacilli viability in the experimental samples 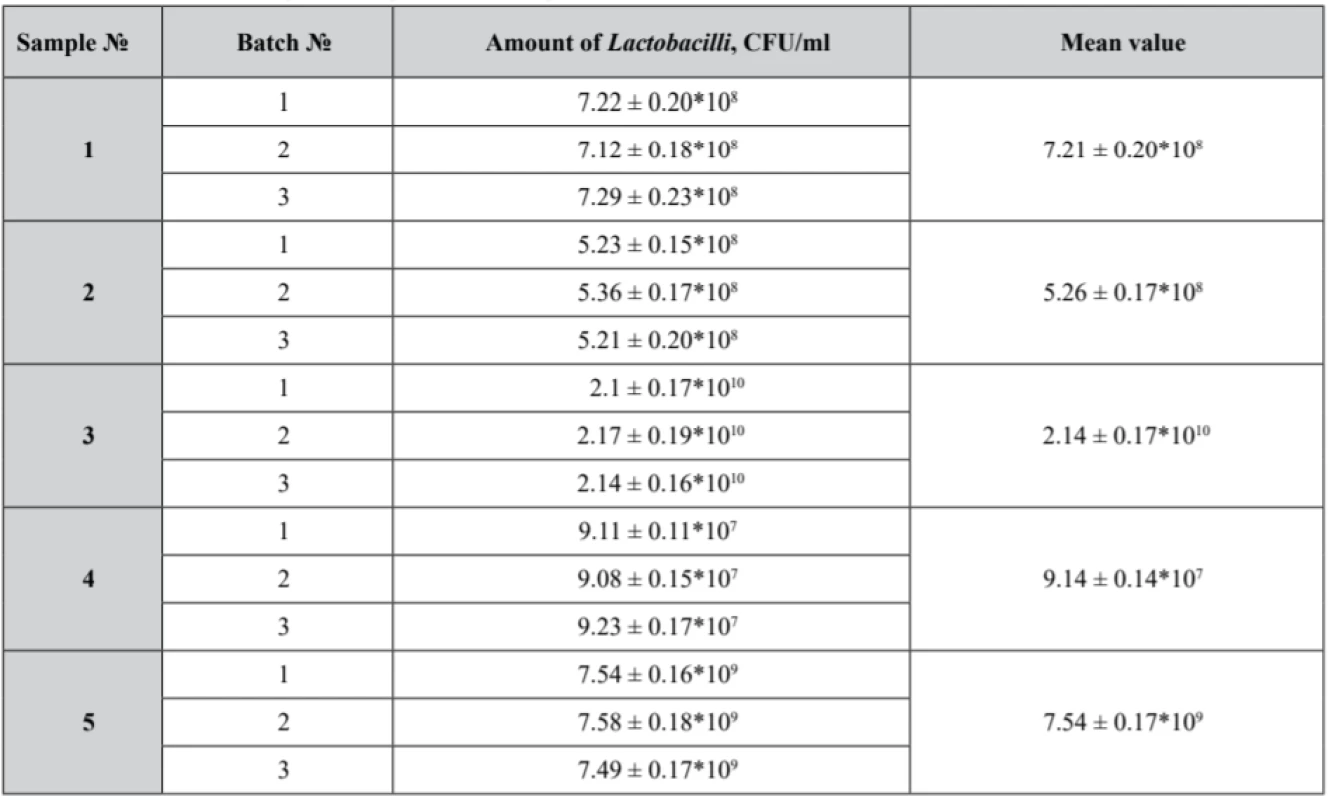
Р ± 95%, n = 5 4. Microbiological purity test results of the experimental samples 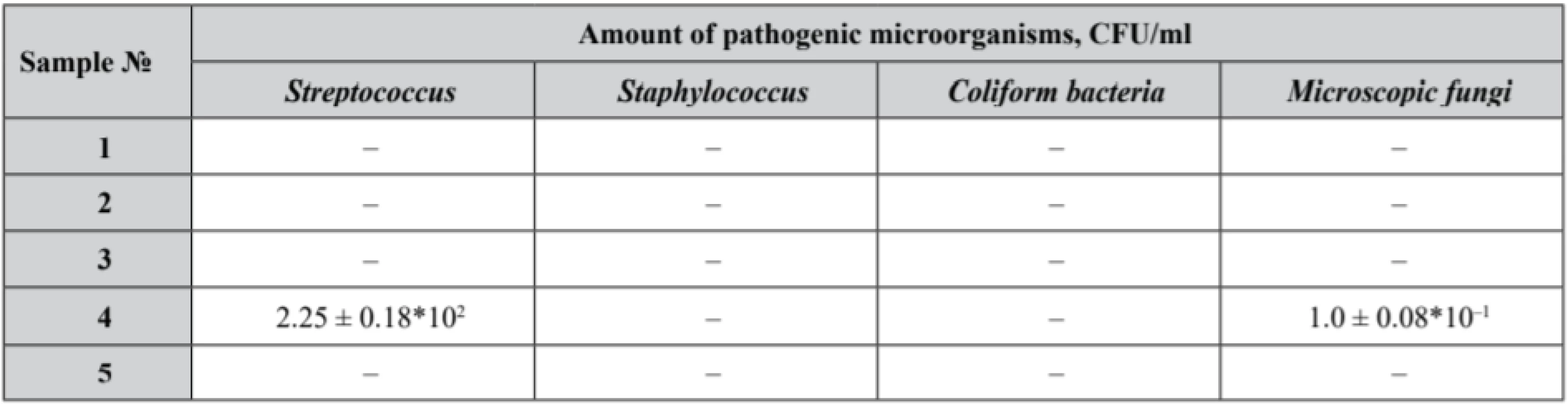
Р ± 95%, n = 5 Efficacy of probiotic microorganisms is determined by appropriate delivery of active substances in reasonable concentrations. From the technological perspective, a probiotic medicine must be composed of particular Lactobacilli strains capable of passing all the technological stages in high living concentrations along with maintaining relevant probiotic activity within the shelf life. Consequently, it is important to consider ranges of probiotic microorganisms in the terms of minimal therapeutic doses53).
According to scientific data, viable Lactobacilli concentrations of 109 CFU for vaginal administration claim their potential to restore and maintain the urogenital tract microflora53).
Our samples № 3 and № 5 complied with the microbiological purity criteria set out and demonstrated satisfactory results with an identified count of Lactobacilli not less than 109 CFU. Lactobacilli assay in samples № 1, № 2 and № 4 did not correspond to the claimed value. Moreover, in sample № 4 the growth of Streptococcus and microscopic fungi was detected.
The obtained results might have been induced either by main physical and chemical properties of the base used or by difference in base components and excipients proportion.
Polysorbate 80 – a well-known non-ionic surfactant and emulsifier – is widely used in food, pharmaceutical and cosmetic (beauty) industry. Moreover, it is utilized as a nutrient medium for Lactobacilli, capable of boosting Lactobacilli growth and contribute to their protection from unfavourable outer factors, including acids, lyophilization, nutrients deficiency, and bile salts influence54). Being included into formulations, polysorbate 80 stimulates production of biologically active substances, namely bacteriocins55) and lactic acid56) that maintain healthy vaginal microbiome.
In addition, due to surfactants hydrophobic bases are much better absorbed by mucous, which is realized by greater interaction of particles surface with mucous57).
What is more, non-ionic surfactant polysorbate 80 use in medicine formulation causes less damage on mucous compared to anionic.
A large number of existing studies in the broader literature have examined the role of polysorbates on substances release. For example, Hanaee et al. research has shown that the release rate of salbutamol in Witepsol® H15-based suppositories altered linearly with the amount of polysorbate 80 in suppository formulations58).
Thus, the results obtained in our research might be interpreted from the perspective of polysorbate presence and concentration in the formulations. Basically, sample № 3 and sample № 5 with polysorbate 80 concentrations in the pessary base up to 2.5–3.0%, demonstrated the highest Lactobacilli count. Sample № 4, with polysorbate concentration up to 2.5%, presented a 107 CFU Lactobacili count. It must be noted that sample № 4 was contaminated with Streptococcus and fungi, being the reason of Lactobacilli growth suppression.
Moreover, diphilic bases have never been used by the authors before for Lactobacilli-containing pessaries formulation. Nonetheless, a number of researches have illuminated the data on probiotic bacteria viability both in hydrophilic and lipophilic based suppositories.
Kaewnopparat et al. investigated L. paracasei HL32 containing solid body and hollow-type suppositories based on Witepsol H-15 or PEGs mixture. The research has shown the identical viability of Lactobacilli from both hydrophilic and lipophilic bases, however the hollow-type suppositories 108 CFU viability was higher than that of 105 CFU solid body ones36).
Rodrigues et al. examined solid body and hollow-type L. acidophilus containing vaginal pessaries. Formulation included Witepsol H-15 or PEGs. All the samples obtained showed a Lactobacilli count of not less than 108 CFU37).
Kale et al. studied three types of pessaries containing lyophilized Lactobacillus spp. based on a PEG mixture. Plain pessaries survival was 105 CFU, while 107 CFU viability was shown by multifunctional bilayer ones and 108 CFU by hollow-type units59).
Pashayan research was devoted to double layer vaginal pessaries with lyophilized L. delbrueckii MH-10 and Achillea millefolium extract powder. All four samples were lipophilic based and indicated 108 CFU viability60).
The results of our study demonstrated the viability of Lactobacilli ranged between 107 and 1010 CFU in all samples. The best value of Lactobacilli assay was identified in sample № 3 which consists of hydrophilic and lipophilic phases in the ratio of almost 1 : 1 and 2.5% of polysorbate 80. Thus, we can confirm that our results show some better viability of Lactobacilli than the subsequences of other researchers.
It is also current to compare the assay results of pessaries and suppositories with other dosage forms for vaginal use. For example, Zárate et al. analyzed gelatin capsules with Lactobacilli. The values of survival are between 107 and 109 CFU37, 53). Fazeli et al. investigated vaginal tablets with Lactobacillus acidophilus and demonstrated viability ranging from 108 to 1010 CFU in different types of vaginal tablets44). These results are in accordance with those of Maggi et al. and Mastromarino et al. whose viability was about 108–109 CFU/ tablet5, 29, 37, 44).
So, the subsequences of other researchers do not object to our results.
The next phase of the research was devoted to experimental samples quality control according to the requirements of the State Pharmacopeia of Ukraine (2nd edition) with results reflected in the Table 5.
5. Experimental samples quality control according to the requirements of the State Pharmacopeia of Ukraine (2nd edition) 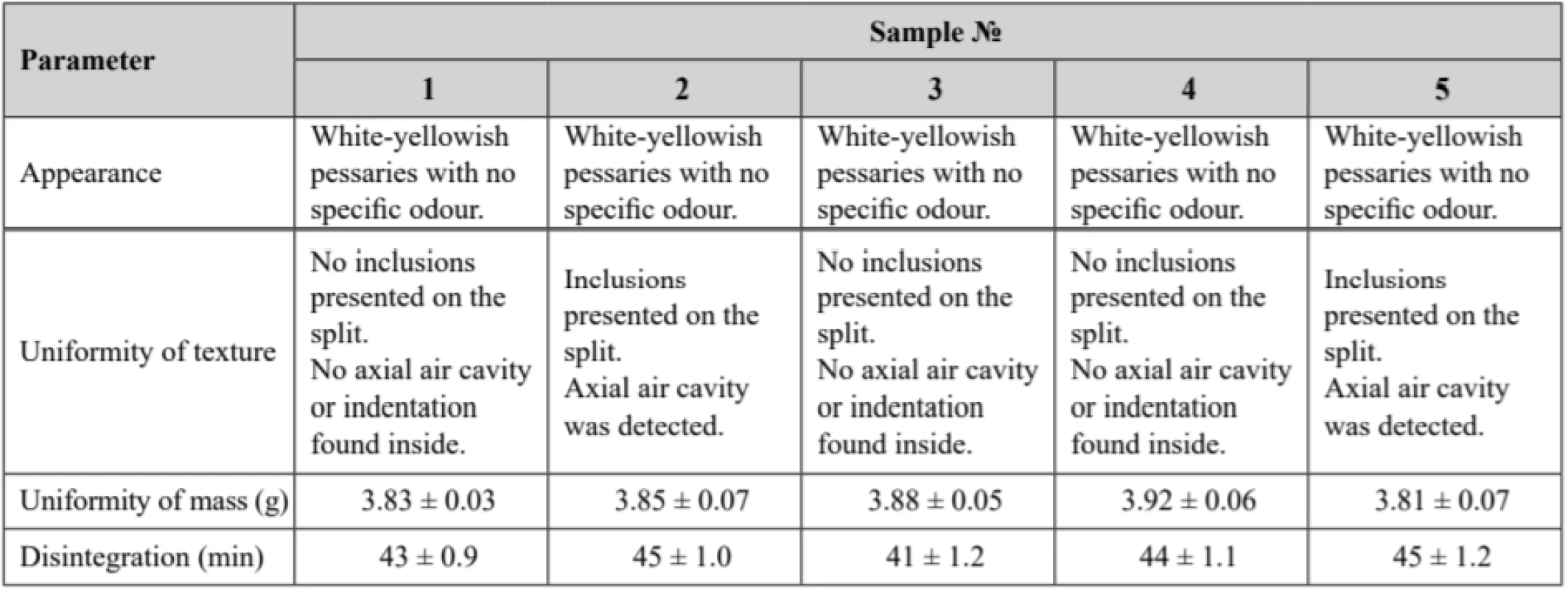
Р ± 95%, n = 5 According to the obtained results, only sample № 2 and sample № 5 did not adequate the requirements of the Uniformity of Texture parameter, since inclusions were detected on the split of the pessaries. The described non-conformity is presumed to be a result of preparation process disruptions, presumably at the moulding phase. Samples № 1, № 3 and № 4 fully complied with the above-mentioned parameter. All samples demonstrated compliance with the requirements of the State Pharmacopeia of Ukraine (2nd edition) on appearance, uniformity of mass, and disintegration.
Solid form disintegration is the first step of substance release61). It should be considered that the disintegration time of sample № 3 was the lowest (41 ± 1.2 min). It might be dependent on the base characteristics, since there was a nearly equal proportion of both hydrophobic and hydrophilic phases (1 : 1) in the sample № 3 formulation, while the hydrophilic phase prevailed in the rest of the samples.
Release of active substance in hydrophilic PEG based pessaries is realized through dissolution, while hydrophobic based pessaries melt62). Thus, melting time of pessaries is shorter than dissolution time.
The experimental samples were also tested for viability of Lactobacillus in prepared samples within the storage period of 6 months at 2–8 °С and at room temperature (25 ± 2 °С) (Table 6).
6. Viability of Lactobacillus in prepared samples after stability tests 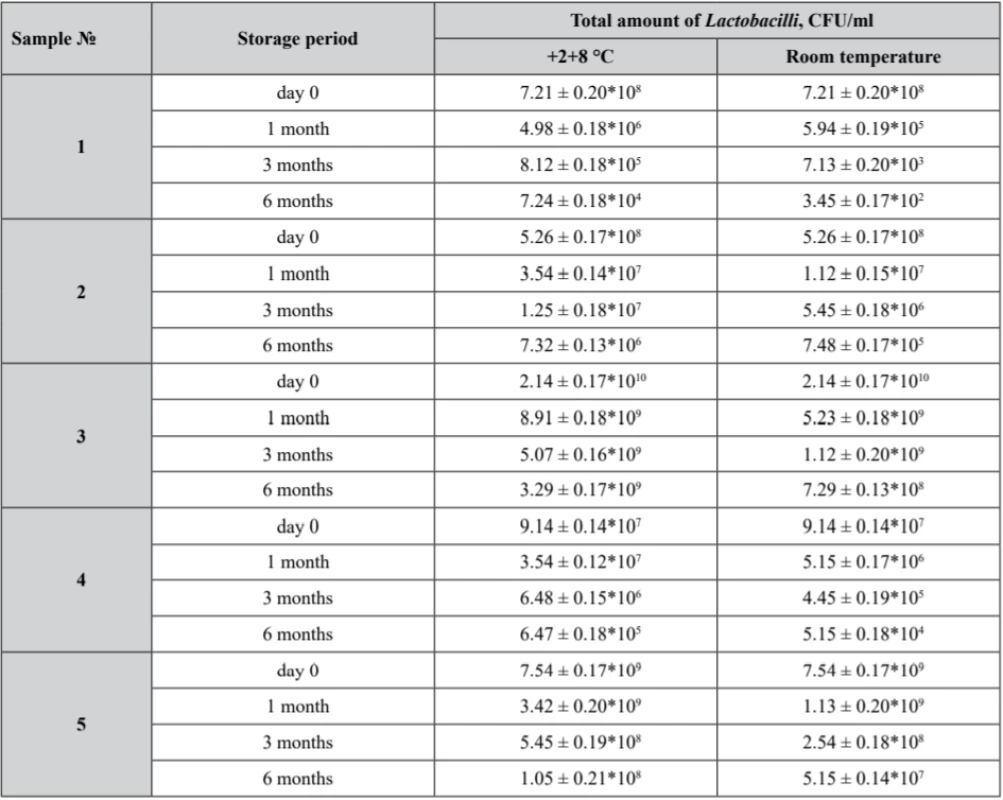
Р ± 95%, n = 5 According to the obtained results, only sample № 3 demonstrated Lactobacilli assay of not less than 109 CFU/ml being stored within 6 months at 2–8 °С.
As it was already stated, samples № 1, № 2 and № 4 Lactobacilli assay did not correspond to the claimed value. In sample № 4 a growth of Streptococcus and microscopic fungi was detected, while samples № 2 failed to comply with the requirements of the Uniformity of Texture parameter.
Nevertheless, according to the data obtained, sample № 3 being stored within 6 months at 2–8 °С showed an appropriate Lactobacilli assay of not less than 109 CFU, while at room temperature it appeared to be 7.29 ± 0.13*108 CFU.
So, it was demonstrated that in all samples the values of Lactobacilli viability decrease during the storage period at both temperature regimes. The more significant reduction of Lactobacilli was observed at the room temperature mode because of the temperature effect.
The similar are the results of research by Pashayan on Lactobacilli viability in suppositories at the temperature of 2–8 °С which demonstrated a decrease in Lactobacilli count in all four samples: within a 6-month storage time the Lactobacilli count decreased from 4.3–5.4*108 CFU to 6.0–9.1*107 CFU60). Kale et al. studies described a decrease in the Lactobacilli count in plain, hollow-type and multifunctional bilayer pessaries during a 1-month storage time. Those results showed a more significant Lactobacilli reduction in plain pessaries at the temperature of 2–8 °С and in all types of pessaries at ambient temperature59).
The study of Kaewnopparat et al. described also a demonstrative decrease in Lactobacilli survival in conventional and hollow-type suppositories on Witepsol H-15 or mixed PEGs during 3 months at ambient temperature than at the temperature of 2–8 °С 36).
But some our samples (№ 3 and № 5) demonstrated a not very high reduction of the Lactobacilli count and showed better results than those in the scientific works of other researchers.
Thus, a significant decrease in Lactobacilli viability is considered to be dependent on the temperature within the storage time36). However, we presume that the decrease in the Lactobacilli count within the shelf life might be related to the nature of the base used, quality of the substance with probiotic activity, and preparation technology.
Sample № 5 also displayed lower Lactobacilli viability not only within shelf life, but also right after preparation. Moreover, sample № 5 did not comply with the requirements of the Uniformity of Texture parameter.
Sample № 3 appeared to be the most promising for further study and development, as it demonstrated strict compliance with the quality parameters set (appearance, uniformity of texture, uniformity of mass, disintegration), along with a satisfactory Lactobacilli count both after preparation and within the 6-months shelf life.
Conclusions
This study was carried out to develop the formulation and technology of vaginal pessaries with probiotic activity for vaginal flora disorders treatment and prophylaxis.
The following formulation was determined as feasible for the investigated dosage form (for 100 g of the base): L. casei ІМВ В-7280 substance for bacteria assay 109 CFU per 1 pessary, PEG-400 – 15.0, PEG-1500 – 25.0, PEG-4000 – 10.0, hard fat – 46.5, polysorbate 80 – 2.5, purified water – up to 100.0. Feasible extemporaneous technology was elaborated for diphilic-based suppositories. Experimental samples proved complete compliance with the requirements for the following parameters: appearance, uniformity of texture, uniformity of mass, and disintegration.
The experimental samples being tested for viability of Lactobacillus right after preparation and within the storage period demonstrated satisfactory stability results for a 6-month shelf life at 2–8 °С. Thus, the results of this study will be used for further development of technological instruction for extemporaneous preparation of vaginal pessaries with probiotic activity.
Acknowledgement
This research was carried out as a part of grand: «Probiotics development for the treatment and prophylaxis infection-inflammatory diseases» (State registration number in Ukraine 0118U005399) and was financed by the Ministry of Education and Science of Ukraine. Research, analysis and paper development were not sponsored by commercial organizations.
Conflicts of interest: none.
Assoc. Prof., DS Pharmacy Zhanna Polova (∗) • S. Aleinyk
O. O. Bogomolets National Medical University,
Department of Pharmacy and Industrial Technology of Drugs
Pushkinskaya str. 22, 01004 Kiev, Ukraine
е-mail: zpolova@ukr.net
A. Kazak
O. O. Bogomolets National Medical University,
Department of obstetrics and gynecology № 1, Kiev, Ukraine
City Clinical Hospital № 18
Sources
1. Mastromarino P., Vitali B., Mosca L. Bacterial vaginosis, a review on clinical trials with probiotics. New Microbiol. 2013; 36(3), 229–238.
2. Kusuma Naik, Avinash, H. Nusrat, L. Krishna, Ravi Kumar. An RCT for efficacy of oral probiotics in treatment of cases with symptomatic white discharge per vagina in rural population. Journal of SAFOG 2012; 4(3), 126–129. doi:10.5005/jp-journals-10006-1193
3. Marcotte H., Krogh Andersen K., Lin Y., Zuo F., Zeng Z., Larsson P. G., Brandsborg E., Brønstad G., Hammarström L. Characterization and complete genome sequences of L. rhamnosus DSM 14870 and L. gasseri DSM 14869 contained in the EcoVag® probiotic vaginal capsules. Microbiol. Res. 2017; 205, 88–98. doi:10.1016/j.micres.2017.08.003
4. Tomusiak A., Strus M., Heczko P., Adamski P., Stefański G., Mikołajczyk-Cichońska A., Suda-Szczurek M. Efficacy and safety of a vaginal medicinal product containing three strains of probiotic bacteria, a multicenter, randomized, double-blind, and placebo-controlled trial. Drug Des. Devel. Ther. 2015; 9, 5345–5354. doi:10.2147/DDDT.S89214
5. Mastromarino P., Brigidi P., Macchia S., Maggi L., Pirovano F., Trinchieri V., Conte U., Matteuzzi D. Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J. Appl. Microbiol. 2002; 93(5), 884–893.
6. Powell A. M., Nyirjesy P. Recurrent vulvovaginitis. Best Pract. Res. Clin. Obstet. Gynaecol. 2014; 28(7), 967–976. doi:10.1016/j.bpobgyn.2014.07.006
7. Bertuccini L., Russo R., Iosi F., Superti F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int. J. Immunopathol. Pharmacol. 2017; 30(2), 163–167. doi:10.1177/0394632017697987
8. Fuochi V., Cardile V., Petronio G., Furneri P. M. Biological properties and production of bacteriocins-like-inhibitory substances by Lactobacillus sp. strains from human vagina. J. Appl. Microbiol. 2019; 126(5), 1541–1550. doi:10.1111/jam.14164
9. Pendharkar S., Brandsborg E., Hammarström L., Marcotte H., Larsson P. G. Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect. Dis. 2015; 3(15), 255. doi:10.1186/s12879-015-0971-3
10. Gaspar C., Donders G. G., Palmeira-de-Oliveira R., Queiroz J. A., Tomaz C., Martinez-de-Oliveira J., Palmeira-de-Oliveira A. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express 2018; 8(1), 153. doi:10.1186/s13568-018-0679-z
11. Petricevic L., Witt A. The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. BJOG 2008; 115(11), 1369–1374. doi:10.1111/j.1471-0528.2008.01882.x
12. Neut C., Verrière F., Nelis H. J., Coenye T. Topical treatment of infectious vaginitis, effects of antibiotic, antifungal and antiseptic drugs on the growth of normal vaginal Lactobacillus strains. Open Journal of Obstetrics and Gynecology 2015; 5(3), 173–180 doi:10.4236/ojog.2015.53024
13. Marcotte H., Krogh Andersen K., Lin Y., Zuo F., Zeng Z., Larsson P. G., Brandsborg E., Brønstad G., Hammarström L. Characterization and complete genome sequences of L. rhamnosus DSM 14870 and L. gasseri DSM 14869 contained in the EcoVag® probiotic vaginal capsules. Microbiol. Res. 2017; 205, 88–98. doi:10.1016/j.micres.2017.08.003
14. Hill D., Sugrue I., Tobin C., Hill C., Stanton C., Ross R. P. The Lactobacillus casei Group, History and Health Related Applications. Front. Microbiol. 2018; 9, 2107. doi:10.3389/fmicb.2018.02107
15. Melgaço A. C., Blohem Pessoa W. F., Freire H. P., Evangelista de Almeida M., Santos Barbosa M., Passos Rezende R., Timenetsky J., Miranda Marques L., Romano C. Potential of maintaining a healthy vaginal environment by two Lactobacillus strains isolated from cocoa fermentation. Biomed. Res. Int. 2018; 30, 7571954. doi:10.1155/2018/7571954
16. Sobel J. D., Schneider J., Kaye D., Levison M. E. Adherence of bacteria to vaginal epithelial cells at various times in the menstrual cycle. Infect Immun. 1981; 32(1), 194–197.
17. Borges S., Silva J., Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014; 289(3), 479–489. doi:10.1007/s00404-013-3064-9
18. Laue C., Papazova E., Liesegang A., Pannenbeckers A., Arendarski P., Linnerth B., Domig K. J., Kneifel W., Petricevic L., Schrezenmeir J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women – a double-blind, randomised, controlled clinical pilot trial. Benef. Microbes 2018; 9(1), 35–50. doi:10.3920/BM2017.0018
19. Homayouni A., Bastani P., Ziyadi S., Mohammad-Alizadeh-Charandabi S., Ghalibaf M., Mortazavian A. M., Mehrabany E. V. Effects of probiotics on the recurrence of bacterial vaginosis, a review. J. Low. Genit. Tract. Dis. 2014; 18(1), 79–86. doi:10.1097/LGT.0b013e31829156ec
20. Kumar N., Behera B., Sagiri S. S., Pal K., Ray S. S., Roy S. Bacterial vaginosis, Etiology and modalities of treatment – A brief note. J. Pharm. Bioallied. Sci. 2011; 3(4), 496–503. doi:10.4103/0975–7406.90102
21. Leyva-Gómez G., Prado-Audelo M. L. D., Ortega-Peña S., Mendoza-Muñoz N., Urbán-Morlán Z., González-Torres M., González-Del Carmen M., Figueroa-González G., Reyes-Hernández O. D., Cortés H. Modifications in Vaginal microbiota and their influence on drug release, challenges and opportunities. Pharmaceutics 2019; 11(5). pii:E217. doi:10.3390/pharmaceutics11050217
22. Balish E., Wagner R. D. Probiotic bacteria for prophylaxis and therapy of candidiasis. Rev. Iberoam. Micol. 1998; 15(4), 261–264.
23. Cadieux P., Burton J., Gardiner G., Braunstein I., Bruce A. W., Kang C. Y., Reid G. Lactobacillus strains and vaginal ecology. JAMA 2002; 287(15), 1940–1941.
24. Anukam K. C., Osazuwa E., Osemene G. I., Ehigiagbe F., Bruce A. W., Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006; 8(12–13), 2772–2776.
25. Mastromarino P., Macchia S., Meggiorini L., Trinchieri V., Mosca L., Perluigi M., Midulla C. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009; 15(1), 67–74. doi:10.1111/j.1469-0691.2008.02112.x
26. Parent D., Bossens M., Bayot D., Kirkpatrick C., Graf F., Wilkinson F. E., Kaiser R. R. Therapy of bacterial vaginosis using exogenously-applied Lactobacilli acidophili and a low dose of estriol, a placebo-controlled multicentric clinical trial. Arzneimittelforschung 1996; 46(1), 68–73.
27. Hallen A., Jarstrand C., Pahlson C. Treatment of bacterial vaginosis with lactobacilli. Sex Transm. Dis. 1992; 19(3), 146–148.
28. Ehrström S., Daroczy K., Rylander E., Samuelsson C., Johannesson U., Anzén B., Pahlson C. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010; 12(10), 691–699. doi:10.1016/j.micinf.2010.04.010
29. Maggi L., Mastromarino P., Macchia S., Brigidi P., Pirovano F., Matteuzzi D., Conte U. Technological and biological evaluation of tablets containing different strains of lactobacilli for vaginal administration. Eur. J. Pharm. Biopharm. 2000; 50(3), 389–395.
30. Burton J. P., Cadieux P. A., Reid G. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl. Environ Microbiol. 2003; 69(1), 97–101.
31. Eriksson K., Carlsson B., Forsum U., Larsson P. G. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta Derm. Venereol. 2005; 85(1), 42–46.
32. Czaja C. A., Stapleton A. E., Yarova-Yarovaya Y., Stamm W. E. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect. Dis. Obstet. Gynecol. 2007; 2007, 35387. doi:10.1155/2007/35387
33. Drago L., de Vecchi E., Nicola L., Zucchetti E., Gismondo M. R., Vicariotto F. Activity of a Lactobacillus acidophilus-based douche for the treatment of bacterial vaginosis. J. Altern. Complement Med. 2007; 13(4), 435–438.
34. Kale V., Trivedi R., Muley P. Proposed design of a dissolution apparatus for vaginal formulations containing probiotics. Dissolution Technologies 2008; 15(2). doi:10.14227/DT150208P27
35. Larsson P. G., Stray-Pedersen B., Ryttig K. R., Larsen S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health 2008; 8, 3. doi:10.1186/1472-6874-8-3
36. Kaewnopparat S., Kaewnopparat N. Formulation and evaluation of vaginal suppositories containing Lactobacillus. International Scholarly and Scientific Research & Innovation 2009; 3(7), 117–120.
37. Rodrigues F., Maia M. J., Neves J., Sarmento B., Amaral M. H., Oliveira M. B. Vaginal suppositories containing Lactobacillus acidophilus, development and characterization. Drug Dev. Ind. Pharm. 2015; 41(9), 1518–1525. doi:10.3109/03639045.2014.963864
38. Falagas M., Betsi G. I., Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 2007; 13(7), 657–664.
39. Ozkinay E., Terek M. C., Yayci M., Kaiser R., Grob P., Tuncay G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG 2005; 112(2), 234–240.
40. Uehara S., Monden K., Nomoto K., Seno Y., Kariyama R., Kumon H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int. J. Antimicrob. Agents 2006; 28(1), S30–34.
41. Kale V. V., Trivedi R. V., Wate S. P., Bhusari K. P. Development and evaluation of a suppository formulation containing Lactobacillus and its application in vaginal diseases. Ann NY Acad. Sci. 2005; 1056, 359–365.
42. Ya W., Reifer C., Miller L. E. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis, a double-blind, randomized, placebo-controlled study. Am. J. Obstet. Gynecol. 2010; 203(2), 120.e1–6. doi:10.1016/j.ajog.2010.05.023
43. Kaewsrichan J., Chandarajoti K., Kaewnopparat S., Kaewnopparat N.. Evaluation of Lactobacilli containing suppository formulation for probiotic use. Mahidol University Journal of Pharm. Sci. 2007; 34(1–4), 1–8.
44. Fazeli M. R., Toliyat T., Samadi N., Hajjaran S., Jamalifar H. Viability of lactobacillus acidophilus in various vaginal tablet formulations. DARU Journal of Pharmaceutical Sciences 2006; 14(4), 172–178.
45. Ashok V. R. Manoj Kumar, D. Murali, Arkendu Chatterjee. A review on vaginal routeas a systemic drug delivery. https://www.researchgate.net/publication/224318371_A_review_on_vaginal_route_as_a_systemic_drug_delivery (18. 10. 2019).
46. Ham A. S., Buckheit R. W. Jr. Designing and developing suppository formulations for anti-HIV drug delivery. Ther. Deliv. 2017; 8(9), 805–817. doi:10.4155/tde–2017–0056
47. Zujkina S. V. Stan ekstemporal`noyi receptury` Ukrayiny` ta problemy` s`ogodennya. Zb. nauk. pracz` spivrobit. NMAPO imeni P. L. Shupy`ka 2018; 32, 294–307.
48. Patent Ukrainy na korysnu model UA № 98881, C12N 1/00 vid 12.05.2015, biul. № 9.
49. Patent Ukrainy na vynakhid UA № 93133, C12N 1/20 vid 10.01.2011, biul. № 1.
50. Ham A. S., Buckheit R. W. Jr. Designing and developing suppository formulations for anti-HIV drug delivery. Ther. Deliv. 2017; 8(9), 805–817. doi:10.4155/tde-2017-0056
51. Rowe R. C., Sheskey P. J., Quinn M. E. Handbook of pharmaceutical excipients. London: Pharmaceutical Press 2009; 517–521.
52. Jankowski A., Dyja R., Sarecka-Hujar B. Dermal and transdermal delivery of active substances from semisolid bases. Indian. J. Pharm. Sci. 2017; 79(4), 488–500. doi:10.4172/pharmaceutical-sciences.1000255
53. Zárate G., Juárez Tomás M. S., Nader-Macias M. E. Effect of some pharmaceutical excipients on the survival of probiotic vaginal lactobacilli. Canadian Journal of Microbiology 2005; 51(6), 483–489. doi:10.1139/w05–031
54. Reitermayer D., Kafka T. A., Lenz Ch. A., Vogel R. F. Interrelation between Tween and the membrane properties and high pressure tolerance of Lactobacillus plantarum. BMC Microbiology 2018; 18(1), 72. doi:10.1186/s12866-018-1203-y
55. Malheiros P. S., Sant’Anna V., Todorov S. D., Franco B. D. G. M. Optimization of growth and bacteriocin production by lactobacillus sakei subsp. Sakei 2a. Brazilian Journal of Microbiology, 2015; 46(3), 825–834. doi:10.1590/S1517–838246320140279
56. Coelho L. F., Lima C. J. B., Rodovalho C. M., Bernardo M. P., Contiero J. Lactic acid production by new Lactobacillus plantarum LMISM6 grown in molasses, optimization of medium composition. Braz. J. Chem. Eng. 2011; 28(1). doi:10.1590/S0104-66322011000100004
57. Realdon N., Dal Zotto M., Morpurgo M., Franceschinis E. Effects of surfactant characteristics on drug availability from suppositories. Pharmazie 2008; 63(6), 459–463. doi:10.1691/ph.2008.7378
58. Hanaee J., Javadzadeh Y., Taftachi S., Farid D., Nokhodchi A. The role of various surfactants on the release of salbutamol from suppositories. Farmaco 2004; 59(11), 903–906. doi:10.1016/j.farmac.2004.07.006
59. Kale V., Patil M., Yadav S. Preparation and evaluation of Novel Vaginal Pessaries of Lactobacilli. Int. J. Drug Dev. & Res. 2012; 4(3), 97–103.
60. Pashayan M. M. Formulation and investigation of vaginal double layer suppositories containing lactobacilli and herbal extracts. The New Armenian Medical Journal 2011; 2, 54–59.
61. Franceschinis E., Realdon N. Effect of the surfactant on the availability of piroxicam as a poorly hydrosoluble drug from suppositories. Pharmazie 2012; 67(1), 37–45. doi:10.1691/ph.2012.1092
62. Chale G. R., Jeughale A. S. Suppository, a review. World Journal of Pharmaceutical Research 2019; 8, 1968–1982.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 2-
All articles in this issue
- Advances in the use of instrumental measurement of colour in the development, production and quality control of drugs, medicinal preparations and pharmaceutical auxiliary substances III
- Nové knihy
- Rosuvastatin-induced rhabdomyolysis due to medication errors
- Evaluation of adherence to treatment in patients suffering from diabetes mellitus
- The influence of «Saprogel» in the wound healing process on rats with a full-thickness wound model
- A cost minimization analysis of α2b-interferon supplementation in complex pharmacotherapy of rotavirus infection in newborns
- Formulation and technology development of vaginal pessaries with probiotic activity
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Evaluation of adherence to treatment in patients suffering from diabetes mellitus
- Rosuvastatin-induced rhabdomyolysis due to medication errors
- Formulation and technology development of vaginal pessaries with probiotic activity
- Advances in the use of instrumental measurement of colour in the development, production and quality control of drugs, medicinal preparations and pharmaceutical auxiliary substances III
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

