-
Medical journals
- Career
Free tensor fascia lata flap – a reliable and easy to harvest flap for reconstruction
Authors: Renuka Sathyamurthy; Nagabhushanaiah Kalapurmat Manjunath; Veena Waiker; Shivalingappa Shanthakumar; Mohan. Swamy Kumara
Authors‘ workplace: Department of Plastic and Reconstructive Surgery, Ramaiah Medical College, Bangalore, India
Published in: ACTA CHIRURGIAE PLASTICAE, 63, 2, 2021, pp. 57-63
doi: https://doi.org/10.48095/ccachp202157Introduction
Flaps are the essence of reconstructive surgery. The type of the defect is a main guide for the choice of a flap. However, certain factors like technique feasibility, duration of the surgery and patient factors do have a role in decision [1]. The advances of microsurgery and better understanding of vascular anatomy has made it possible to reconstruct the defect nearly to the normal. The primary type of a free flap (whether muscle/fasciocutaneous flap) is dictated by the defect or the wound characteristics. However, the choice of a flap (tensor fascia lata – TFL, anterolateral thigh flap – ALT, thoracodorsal artery perforator – TAP) depends on various factors like the component of the flap, pedicle length required, the ease of harvest and donor site morbidity [2]. Tensor fascia lata (TFL) is one of the myocutaneous flaps which has very constant pedicle anatomy and well-developed components other than a muscle [3]. Hence, harvesting the flap is less time-consuming compared to other flaps. When used as a free flap, it has many advantages, too. This study was undertaken to assess the success outcome of free TFL flaps.
The aim of the presented study was to ascertain success rate of reconstruction of various defects with tensor fascia lata free flap. The objectives were to determine the ease of harvest of the flap in terms of time to harvest the exact location of the pedicle and to determine the outcome of the free TFL flap in terms of flap loss.
Materials and methods
Group of patients
The hospital based prospective study was carried out at the Department of Plastic and Reconstructive Surgery, over a period of 24 months between November 2016 and November 2018. The subjects were enrolled according to the inclusion and exclusion criteria after obtaining an informed consent (Tab. 1).
1. Demographic profile of the patients. 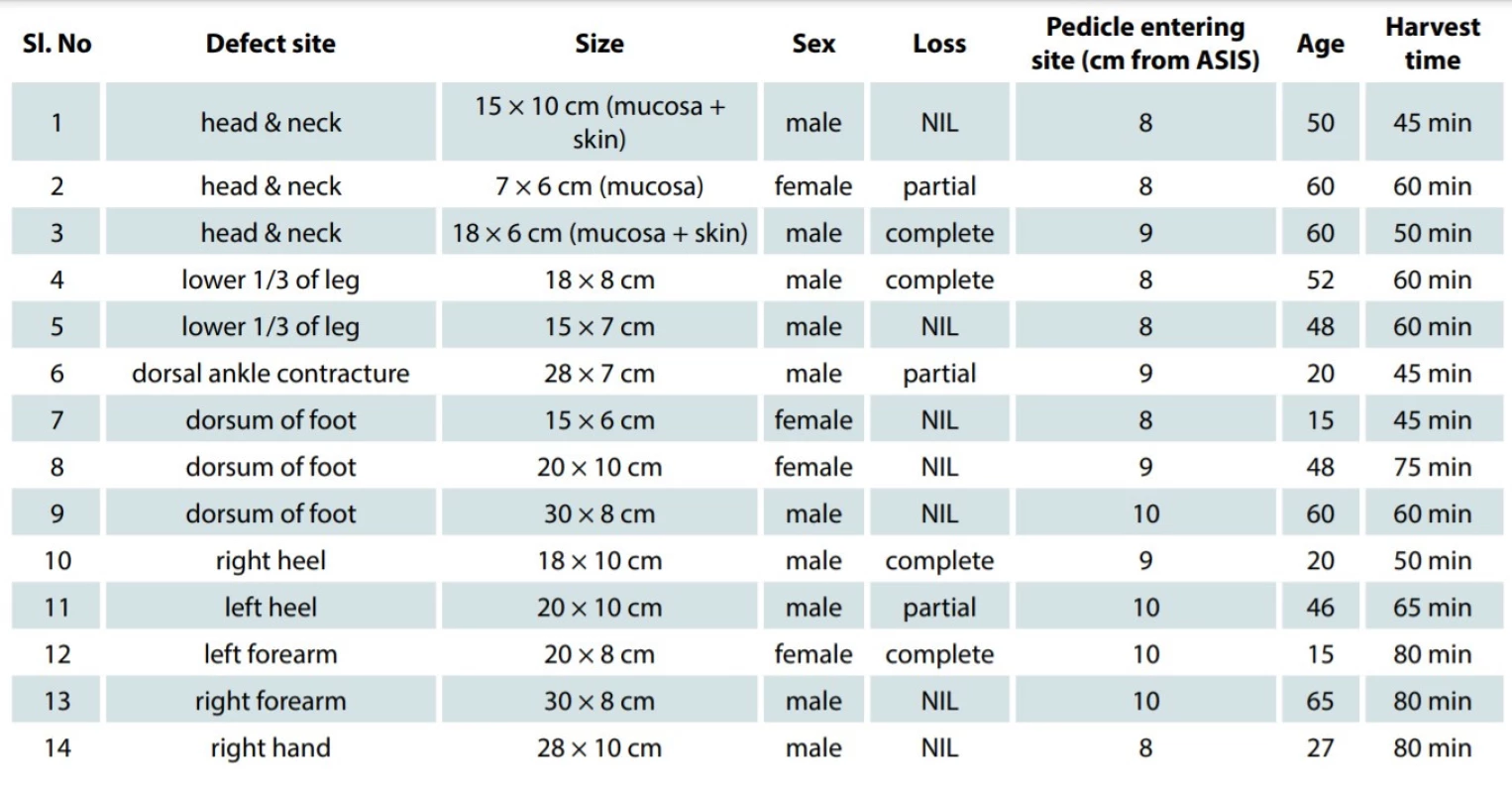
ASIS - anterior superior iliac spine, NIL - no loss of flap The inclusion criteria included a composite defect in different regions of the body, age group 12–70 years, and a good Doppler flow in the recipient vessels.
The exclusion criteria included patients unfit for the free flap surgery with cardiopulmonary disease, renal failure, blood coagulation disorders and peripheral vascular diseases. A TFL free flap was planned in the defects which warranted a fasciocutaneous flap and in whom an ALT fasciocutaneous perforator was absent in the Doppler study, too.
Flap elevation technique
The musculocutaneous flap includes the entire muscle and an island of overlying skin of the size 10 × 20 cm, larger than the muscle itself. The incision is made along the distal border of the flap and is continued through the fascia lata. Then the anterior and posterior incisions are made, extending from below upwards and toward the anterior superior iliac spine and the border of iliac crest. The dissection is continued deep to the fascia lata overlying the vastus lateralis rapidly in a relatively bloodless field. At a level of 8–10 cm below the anterior superior iliac spine (Fig. 1), the terminal branches of the lateral circumflex femoral artery are easily identified. The descending branch courses distally either between the vastus lateralis and the tensor fascia lata or within the vastus lateralis itself. The transverse branch enters the tensor fascia lata on its deep medial surface and is identified easily. The dissection is carried out proximally and separated from gluteal muscles. The follow up was done daily till the wound healed and later at 2 - and 6-month intervals after the discharge of the patient.
Fig. 1. Pedicle surface marking. 
Donor site management
Since the flaps harvested were large in size, the majority of flaps required skin grafts for donor site coverage. In cases where primary closure was possible, the donor site was closed primarily.
Results
There were totally14 patients reconstructed with free TFL flaps. Out of the 14 patients, 10 (71%) and 4 (29%) were below and above 60 years of age, respectively. Ten were male patients and 4 were female patients. Eight patients (58%) had haemoglobin levels above 12% before reconstruction. In our study, we used free tensor fascia lata myocutaneous flaps for the coverage of composite defects following excision of buccal mucosal carcinoma in 3 patients, leg defects in 2 patients, dorsum of foot defects in 4 patients, heel defects in 2 patients, and upper limb defects in 3 patients. The anatomic location of the defect was more often on the lower limbs – 8 cases (58%), followed by the upper limb and the head and neck area (3 cases each 21%) (Fig. 2). The mean flap harvest time was 62.07 (45–80) min. The mean size of the pedicle entry was 8.7 cm (8–10) cm from the anterior superior iliac spine. The minimum and maximum lengths of the flap harvest were 14 and 30 cm, respectively. Type 2 diabetes mellitus was a comorbidity in 7 (50%) of our patients; 1 patient had thrombocytosis. Out of the 14 flaps, 10 (71%) of them were successful completely and 4 (29%) of them had partial loss. Out of the 4 patients who had partial flap loss, 3 had diabetes mellitus and 1 had pre-operative thrombocytosis. On re-exploration, one patient had arterial thrombosis, which was re-anastomosed and, eventually, the patient had only minimal loss of the flap. The defect was covered by a skin graft. All the donor sites healed well and the patients had no deficit in hip extension or during walking.
Fig. 2. Recipient site distribution. 
Discussion
Tensor fascia lata is a flat muscle which acts as an accessory flexor and medial rotator of the thigh. Its arterial supply is from the transverse branch of the lateral femoral circumflex iliac artery and venous drainage from the pair of venae commitantes [4]. In the study on a hundred of cadavers, it is inferred that the muscle is supplied by a single dominant transverse branch in 67% and by anterior branch in 13%; in 20% of cases, it has additional supply from the common femoral or medial circumflex femoral vessels. The vascular pedicle enters the muscle approximately 8 cm distal to the anterior iliac spine [5]. In our cadaver study, there were 10 TFL flaps studied and the pedicles were identified at the average 9.15 (8–10.5) cm. Cadaver studies show that the blood supply to the skin is provided by numerous perforators from the underlying muscular arteries; the artery after piercing the muscle pierces the fascia and divides superiorly and inferiorly to supply the skin. Sometimes it is supplemented by the branches of the descending branch of lateral circumflex femoral vessels. The vascular pedicle is very consistent in position. It enters the muscle at 8 cm from the anterior superior iliac spine (ASIS) [6]. The diameter of the pedicle of TFL flap was measured to be 1.5–2 mm. The calibre of the vessels is compatible for microvascular anastomosis. This makes the flap ideal for microvascular surgery. The vascular pedicle in this flap can be dissected to 4–5 cm in length, which is considered relatively short by some surgeons. However, the length of the pedicle can be increased up to 10 cm, if the descending branch of lateral circumflex femoral and rectus femoris branch are divided [6]. The dissection up to the lateral circumflex and ligating ascending branch and descending branch adds additional 4–5 cm to the pedicle length and also increases the diameter to 2–3 mm. Although this myocutaneous flap has skin, subcutaneous tissue, fascia and muscle, it is a thinner and more pliable flap compared with an ALT free flap [7]. In our study, 3 patients with composite defects of the head and neck were reconstructed using tensor fascia lata flap. In all these patients, the muscle was debulked completely, to make the flap thinner. Since the flap was completely thin, the flap contoured well to the defect. Out of 3 patients, 1 patient had partial loss of the flap (inner lining). The patient had peroperative hypotension and was put on noradrenaline support perioperatively. This led to a perioperative fluctuation of blood pressure. During revision surgery, the patient had huge external clot pressing on the anastomosis leading to venous thrombosis. The clot was evacuated and venous re-anastomosis was performed. Eventually the inner lining of the flap necrosed. The outer skin paddle was healthy and inner lining left for secondary healing. The majority of the studies conclude that an ALT flap is considered as an ideal soft tissue donor for head and neck reconstruction [8–10] but the dissection of the musculocutaneous perforator is a challenge [11–13]. Moreover, the inconsistent anatomy of perforators make it difficult to harvest sometimes. In patients with large amounts of subcutaneous fat, the flap harvested is too bulky for head and neck reconstruction.
In all 3 of our cases, preoperative Doppler studies could not identify the perforator for an ALT flap. In all 3 cases, a TFL was harvested within 60 minutes, which is its greatest advantage (Fig. 3A–D). The flap harvested was thin and contoured the buccal mucosa. In one of the patients, we chose the free tensor fascia lata flap for buccal mucosa reconstruction, mainly because the flap territory was thin and non-hairy compared with other flap sites. In the study conducted by Subramania Iyer et al., the flap was also harvested along with the iliac crest for maxillary and orbital reconstruction with a good outcome [14]. For head and neck reconstruction, a TFL provides an alternative to an ALT.
Fig. 3A. Preoperative photo of the patient with carcinoma of buccal mucosa. 
Fig. 3B. Composite defect after excision of the tumour. 
Fig. 3C. Status after the inset of free TFL flap. 
Fig. 3D. Late postoperative result. 
In 8 of our cases, leg and foot reconstruction was performed with a TFL flap. In these cases, the defects were large. An ALT provides a wide territory to harvest large flaps. In all these cases, perforators were not located in preoperative Doppler studies. Studies by Contendin [7] showed that in the absence of an ALT perforator, a TFL perforator flap can be harvested. They also suggested that the skin in a TFL perforator flap can be of large dimensions [15–17]. Kardbeg et al. [18] harvested a TFL flap up to 30 × 15 cm as a pedicled flap. However, no studies have defined the exact boundaries of the skin territory in these flaps [4]. In our study, we used a free tensor fascia lata flap to resurface two composite heel defects. In these cases, fascia lata adhered well to the calcaneum, providing a durable cover for the heel defect. Two leg and four foot dorsum defects were resurfaced with a free TFL flap as well (Fig. 4A–C). In 3 of our patients, there was a minimal flap necrosis. One patient was a diabetic and developed arterial thrombosis within 12 hours of the anastomosis. The patient had atherosclerotic vessels; he had a good flow immediately after the anastomosis but later developed arterial thrombosis. The other patient was a young male. The muscle component of the flap was bulky, the cut ends exerted profuse bleeding postoperatively, leading to pressure effects on the anastomosis and venous thrombosis. In both cases, timely re-exploration salvaged the flap; however, there was a flap necrosis of a minimal extent. This minimal necrosis of the flap required regular dressing and the wound healed by secondary intention.
Fig. 4A. Posttraumatic foot defect with exposed metatarsal bone. 
Fig. 4B. Immediate result after the inset of the flap. 
Fig. 4C. Late result after reconstruction. 
In 3 of our cases, a TFL was used for hand and forearm reconstruction. During the flap harvest, we found that the flap anatomy was constant and the pedicle was found at 8–10 cm from the ASIS in all 3 cases. In 2 cases, the different components of the flaps were used for different purposes. In one case, the fascia lata was used for bridging the gaps of the excised tendons and the flap provided an easy gliding surface. In another case, the patient had necrosis of the flexor group of the muscles exposing the bone. In this case, the TFL muscle was used to cover the defect and the fascia was used to bridge the tendon gaps. Same patient had a circumferential loss of the soft tissue over the metacarpals and phalanges. To cover this defect, a large skin paddle was harvested and used for resurfacing the defect. All fingers except the thumb were resurfaced on both ventral and dorsal side. The fingers moved as one unit (Fig. 5A–C). In these cases, the defect size was smaller but required a thin pliable flap, which also allowed easy gliding of the tendons underneath. By doing so, we could reconstruct tendons and cover the defect in a single stage. In each site of the reconstruction, the distinctive advantage of a TFL flap was utilised.
Fig. 5A. Preoperative photo of hand and forearm defect. 
Fig. 5B. Free TFL flap used for bridging the tendon gap. 
Fig. 5C. Status after the inset of free TFL flap. 
Free flap and microvascular surgery have their own limitations like a long operating time and a high anaesthetic risk [19]. They represent indirect implications on the hospitalisation costs. The majority of a microvascular surgery time is spent on flap harvest. In our study, all flaps are harvested along with the muscle and hence were harvested within 1 hour, compared to ALT, which was harvested in 1½ hour, where the time is spent more on dissecting an intramuscular pedicle. This is a major advantage as it reduces the total operating hours. Akthar et al. [20] also concluded in their study that a TFL is best suited for critical patients who cannot tolerate a long operating time. Besides this, the flap has a reliable, highly consistent vascular pedicle, which can support a large dimension flap [21]. In our study, flap was harvested with the muscle. Retrospectively, we think this was the cause of flap loss in 2 cases. As per the literature, isolation of a perforator through the muscle was also possible in a short time. Reducing the muscle bulk can improve a success rate. An ALT is proved beyond doubt as a first choice for soft tissue reconstruction. Nevertheless, anatomical inconsistency of the vascular pedicle is a major hurdle while harvesting the flap. Compared with an ALT, a tensor fascia lata free flap has a certain disadvantage like a short pedicle length [16,22] but certain manoeuvres like dissecting proximally up to lateral circumflex femoral artery (Fig. 6) can increase the pedicle length. Studies also show that the calibre of a TFL pedicle is good for survival of a large flap and for its metabolic demands [23]. Hence, a free TFL can be used either as a first choice or as an alternative to an ALT flap wherever a musculocutaneous ALT perforator is inconsistent.
Fig. 6. Dissection of the pedicle up to lateral circumflex femoral branch. 
Conclusion
Tensor fascia lata is a thin, pliable, myocutaneous flap, which can be used either as a first choice or as an alternative to an ALT fasciocutaneous flap. The consistent anatomy of a vascular pedicle is advantageous in a free TFL, compared to an ALT flap. Although the pedicle length is short, it can be increased when dissection is continued up to the lateral circumflex femoral artery. An excess muscle bulk proves hazardous in some situations. Reducing the muscle bulk around the vascular pedicle prevents undue pressure on the anastomosis, and thus improves a success rate. The non-hair bearing skin in the TFL flap territory makes it a choice for buccal mucosa reconstruction. Similarly, the use of a fascial component for tendon reconstruction can avoid the second stage surgery in hand and forearm reconstruction. The harvest time is very short compared to any other flaps and hence makes it the flap of choice in patients who are critical and cannot withstand a long operating time.
Roles of authors: All authors have been actively involved in planning, preparation, analysis and interpretation of the findings as well as in enactment and processing of the article with the same contribution.
Declaration: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. An informed consent for being included in the study was obtained from all patients.
Conflict of interest: There is no conflict of interest to disclose.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for profit sectors.
K. N. Manjunath, MS, Mch
Department of Plastic and Reconstructive Surgery
Ramaiah Medical College
M S Ramaiah Nagar, MSRIT Post
Bangalore 560 054, India
e-mail: drknmanjunath@gmail.com
Submitted: 25. 02. 2021
Accepted: 09. 05. 2021
Sources
- Aladimi MT., Han B., Li C., et al. Factors to consider when deciding on the type of free-flap reconstruction of head and neck soft tissue defects. J Otorhinolaryngol Relat Spec. 2017, 79 : 230–238.
- Broome Martin M., Juilland N., Litzistorf Y., et al. Factors influencing the incidence of severe complications in head and neck free flap reconstructions. Plast Reconstr Surg – Global Open. 2016, 4 : 1013
- Shestak KC., Edington HJD., Johnson RR. The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: Anatomy, surgical technique, applications, and limitations revisited. Plast Reconstr Surg. 2000, 105 : 731–739.
- Rifaat MA., Gawad WSA. The use of tensor fascia lata pedicled flap in reconstructing full thickness abdominal wall defects and groin defects following tumor ablation. J Egypt Natl Canc Inst. 2005, 17 : 139–148.
- Saadeh FA., Haikal FA., Abdel-Hamid FA. Blood supply of the tensor fasciae latae muscle Clin Anat. 1998, 11 : 236–238.
- Williams JK., Carlson GV., DeChalain T., et al. Role of tensor fasciae latae in abdominal wall reconstruction. Plastic and Reconstructive Surgery. 1998, 101 : 713–718.
- Contedini F., Negosanti L., Pinto V., et al. Tensor fascia latae perforator flap: An alternative reconstructive choice for anterolateral thigh flap when no sizable skin perforator is available. Indian J Plast Surg. 2013, 46 : 55–58.
- Coskunfirat OK., Ozkan O. Free tensor fascia lata perforator flap as a backup procedure for head and neck reconstruction. Ann Plast Surg. 2006, 57 : 159–163.
- Chen HC., Tang YB. Anterolateral thigh flap: An ideal soft tissue flap. Clin Plast Surg. 2003, 30 : 383–401.
- Chana JS., Wei FC. A review of the advantages of the anterolateral thigh flap in head and neck reconstruction. Br J Plast Surg. 2004, 57 : 603–609.
- Engel H., Gazyakan E., Cheng MH., et al. Customized reconstruction with the free anterolateral thigh perforator flap. Microsurgery. 2008, 28 : 489–494.
- Wei FC., Jain V., Celik N., et al. Have we found an ideal soft tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002, 109 : 2219–2226.
- Wei FC., Celik N., Jeng SF. Application of “simplified nomenclature for compound flaps” to the anterolateral thigh flap. Plast Reconstr Surg. 2005, 115: 1051–1055.
- Iyer S., Kuriakose MA. Tensor Facia Lata-iliac crest osteocutaneous flap for orbitomaxillary reconstruction: A preliminary report. Indian J Plast Surg. 2010, 43 : 8–13.
- Deiler S., Pfadenhauer A., Widmann J., et al. Tensor fasciae latae perforator flap for reconstruction of composite Achilles tendon defects with skin and vascularized fascia. Plast Reconstr Surg. 2000, 106 : 342–349.
- Koshima I., Urushibara K., Inagawa K., et al. Free tensor fasciae latae perforator flap for the reconstruction of defects in the extremities. Plast Reconstr Surg. 2001, 107: 1759–1765.
- Coskunfirat OK., Ozkan O. Free tensor fascia lata perforator flap as a backup procedure for head and neck reconstruction. Ann Plast Surg. 2006, 57 : 159–163.
- Karabeg R., Dujso V., Jakirlic M. Application of tensor fascia lata pedicled flap in reconstructing trochanteric pressure sore defects. Medicinski arhiv. 2008, 62 : 300–302.
- Krishnan KG., Winkler PA., Müller A., et al. Closure of recurrent frontal skull base defects with vascularized flaps – a technical case report. Acta Neurochirurgica. 2000, 142 : 1353–1358.
- Akhtar MS., Khurram MF., Khan AH. Versatility of pedicled tensor fascia lata flap: a useful and reliable technique for reconstruction of different anatomical districts. Plast Surg Int. 2014. 2014 : 846082.
- Hubmer MG., Schwaiger N., Windisch G., et al. The vascular anatomy of the tensor fasciae latae perforator flap. Plastic and Reconstructive Surgery. 2009, 124 : 181–189.
- Kimura N., Satoh K., Hosaka Y. Tensor fasciae latae perforator flap. Clin Plast Surg. 2003, 30 : 439–446.
- Tursun R., Marwan H., Marshall Green J., Alotaibi F., LeDoux A. Combined anterolateral thigh and tensor fasciae latae flaps: an option for reconstruction of large head and neck defects. Oral Maxillofac Surg. 2017, 75 : 1743–1751.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2021 Issue 2-
All articles in this issue
- Editorial
- Long-term donor-site morbidity after thumb reconstruction with twisted-toe technique
- Supraclavicular artery island flap for head and neck reconstruction
- Free tensor fascia lata flap – a reliable and easy to harvest flap for reconstruction
- Presence of circulating tumor cells in a patient with multiple invasive basal cell carcinoma - a case report
- Pyoderma gangrenosum: a rare complication of reduction mammaplasty – a case report
- Negative pressure therapy in the orofacial region in oncological patients – two case reports
- Thirty-five years of the Department of Plastic Surgery and Burns Treatment at the University Hospital in Hradec Králové
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Pyoderma gangrenosum: a rare complication of reduction mammaplasty – a case report
- Free tensor fascia lata flap – a reliable and easy to harvest flap for reconstruction
- Supraclavicular artery island flap for head and neck reconstruction
- Long-term donor-site morbidity after thumb reconstruction with twisted-toe technique
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

