-
Medical journals
- Career
Factors related to maternal mortality rate in covid-19 patients – a cross-sectional study from an Indonesian covid-19 referral hospital
Authors: S. Azizah; A. P. Mustika; N. Widjaya; R. Irwinda; A. K. Harzif; T. Priyatini; M. Maidarti; M. A. F. Dilmy; V. Silvana; C. Teguh; A. T. Rivai; Z. Djuliannisaa; V. A. F. Nasution; D. L. Darmestari; R. Sukmadewanti; Z. T. Cahyaningrum; E. S. N. Amalia; M. Jamilah
Authors‘ workplace: Universitas Indonesia Hospital, Indonesia
Published in: Ceska Gynekol 2023; 88(5): 334-346
Category: Original Article
doi: https://doi.org/10.48095/cccg2023334Overview
Background: The covid-19 pandemic may cause severe clinical manifestations in a vulnerable population, such as pregnant women. Based on Indonesian Obstetrics and Gynecology Association (POGI), the number of maternal deaths due to covid-19 from April 2020 to April 2021 reached 3% and increased to 9% since the delta variant of covid-19 emerged. This research was expected to identify factors that are related to the mortality rate of pregnant women with covid-19. Materials and methods: This was a cross-sectional study using secondary data collected from June 2020 to August 2021. The study was conducted in Universitas Indonesia Hospital, a national covid-19 referral hospital. Patient characteristics, pregnancy profile, comorbidities, laboratory results, chest X-ray examination, treatment options, and the severity of symptoms were evaluated. In addition, bivariate data analysis was carried out using the SPSS device. Results: Out of 114 research subjects, seven patients (6.1%) died, and 107 patients (93.9%) survived. The risk of mortality was significantly (P < 0.05) related to patients’ age, duration of hospitalization, gestational age, severity rate of covid-19, the level of hemoglobin, leukocyte count, platelet count, lymphocytes, the levels of D-dimer, C-reactive protein, transaminase enzymes, urea, creatinine, eGFR, sodium, potassium, and procalcitonin. In addition, significant differences (P < 0.05) related to maternal mortality rate were also shown in the presence of comorbidities (type 2 diabetes, congestive heart failure, coronary artery disease/acute coronary syndrome, and urinary tract infection), and the use of steroids and tocilizumab. Conclusion: Various factors significantly related to the mortality rate of pregnant women with covid-19. This study may become the basis for a further study with a larger number of subjects, adjustment of assessment and management of covid-19 infected pregnant women, thus hopefully reducing the risk of mortality in pregnant women with covid-19.
Keywords:
mortality – COVID-19 – pregnancy – related factors
Background
On March 11, 2020, WHO declared the Coronavirus Disease (covid-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS--CoV-2) [1]. Covid-19 infected more than 100,000,000 people worldwide. Until now, more than two million people have died. This virus is considered hazardous for a vulnerable population, including pregnant women [2]. Indonesian Obstetrics and Gynecology Association (POGI – Perkumpulan Obstetri dan Ginekologi Indonesia) reported that 536 pregnant women were infected with covid-19 and the mortality rate was up to 3% from April 2020 to April 2021. Its rate increased three times when the delta variant of covid-19 emerged [3]. A study showed that the mortality rate of pregnant women infected with covid-19 was 22 times higher than it was in the general population [4]. The study showed that pregnant women were highly affected by covid-19 infection.
During pregnancy, the body of a pregnant woman will undergo several physiological changes such as reduced residual function volume, higher diaphragm position, respiratory tract mucosal edema, and immune system changes. The changes may increase the susceptibility of covid-19 infection and exacerbate the symptoms [5]. As a result, numerous complications may occur, such as respiratory failure, fetal distress and premature rupture of the membrane or contraction. Furthermore, neonatal asphyxia and perinatal death can occur as a result. The higher mortality rate in pregnant women can be explain by the physiological changes. Since covid-19 is a new emerging disease, the factors related to maternal death in pregnant women infected with covid-19 have not yet been understood [2].
Universitas Indonesia Hospital (RSUI – Rumah Sakit Universitas Indonesia) is a national referral hospital for covid-19 located in Depok, West Java. RSUI had a negative pressure operating room which was used when surgeries had to be performed on patients who had covid-19. This room was used to contain airborne contaminants within the room. The availability of the negative pressure operating room made Universitas Indonesia Hospital a referral hospital for pregnant women infected by covid-19 who were likely to require termination of pregnancy.
Adhering to the high mortality rate of pregnant women infected by covid-19 which continues to increase, the factors and probable mechanisms associated with mortality should be identified immediately so that hopefully several measurements or early intervention can be recommended for pregnant women infected by covid-19, even within limited resources.
Materials and methods
This was a cross-sectional study. The data were taken from electronic medical records from June 2020 to August 2021. We included all pregnant women who were confirmed of having covid-19 infection using SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) test. The extracted data included patient characteristics such as maternal age, gestational age, pregnancy profile, comorbidities, laboratory results, chest X-ray examination, and outcomes.
The characteristics of the research subjects included maternal age, duration of hospitalization, gestational age, severity degree of covid-19, comorbidities, radiology results, laboratory results, empirical antibiotics, antiviral therapy, steroid therapy, tocilizumab therapy, pregnancy outcomes and mortality outcomes. The severity degree of covid-19 infection was categorized to mild-moderate and severe-critical covid-19 infection. Comorbidities which were evaluated were type 2 diabetes mellitus (DM), chronic hypertension, preeclampsia, eclampsia, chronic heart failure (CHF), coronary artery disease (CAD) /acute coronary syndrome (ACS), asthma, and urinary tract infection (UTI). Mortality outcomes of covid-19 infection were described as died or survived.
The data were analysed using SPSS software version 20. Distribution of the data was analysed using the Kolmogorov Smirnov test. Normally distributed data then presented as mean ± SD, meanwhile the data that were not normally distributed were presented as the median (min-max). Then, numerical data were analysed using One Way ANOVA or unpaired T-test, in addition to the Mann Witney or Kruskal Wallis tests. Meanwhile, categorical data were analysed using the Chi-square or Fischer test.
The proposal of this study was approved by the Ethics Committee of Universitas Indonesia Hospital under the approval number S-068/KETLIT/RSUI/XI/2021 and protocol number 2021-09-106. We also followed The Declaration of Helsinki guidelines.
Results
About 114 covid-19 patients were included in the study with 7 (6.1%) patients that died and 107 (93.9%) patients that survived. The mean age of the patients was 30 years old. About 95 patients (83.3%) underwent hospitalization for less than 14 days, with a median of 10 days and the longest duration of 41 days. The severity degree of covid-19 was dominated by mild-moderate symptoms (79.8%). The most common comorbid was UTI (25.4%). The chest X-ray examination showed infiltrates in 85 patients (77.2%). Empirical antibiotics were given to 102 patients (89.5%), antiviral therapy to 76 patients (66.7%), steroid therapy to 57 patients (50%), and tocilizumab therapy to 110 patients (3.5%). The median gestational age was 37 weeks.
The median hemoglobin (Hb) level was 11.4 g/dL. The median leukocyte level was 9,100/µL, while the highest level was 67,300/µL. The mean value of D-dimer was 1951.9 ng/mL. The mean value of C-reactive protein (CRP) was 41.1 mg/L, while the mean value of interleukin-6 (IL-6) was 67.2 pg/mL. The estimated glomerular filtration rate (eGFR) had a median of 124.6 mL/min/1.73 m2. Procalcitonin levels had a median value of 0.20 ng/mL, with highest value of 36.80 ng/mL.
Factors that were significantly related to the outcome (P < 0.05) were the patients’ age, duration of hospitalization, gestational age, severity degree of covid-19, type 2 diabetes mellitus, CHF, CAD/ACS, hemoglobin levels, leukocytes, platelet count, ESR, lymphocytes, neutrophils, D-dimer, CRP, urea, creatinine, eGFR, SGOT, SGPT, sodium, potassium, procalcitonin, presence of a UTI, steroid and tocilizumab therapy.
The laboratory examination was not performed for all patients. Data analysis was only performed on a number of these patients. ESR level was obtained from 68 patients. Prothrombin time (PT) and activated partial thromboplastin time (APTT) values were examined in 57 patients. IL-6 level was only obtained from 16 people with a p-value of 0.515. Blood electrolyte levels in the form of sodium, potassium, and chloride were obtained from 68 patients, but only sodium and potassium levels were significantly related to the outcome. BGA was obtained from 78 patients where the results were classified as acidosis/alkalosis and normal. Empirical antibiotic and antiviral therapies were not significantly related to the outcome (Tab. 1).
1. Characteristics, clinical data and outcomes.Tab. 1. Charakteristika, klinická data a výsledky. 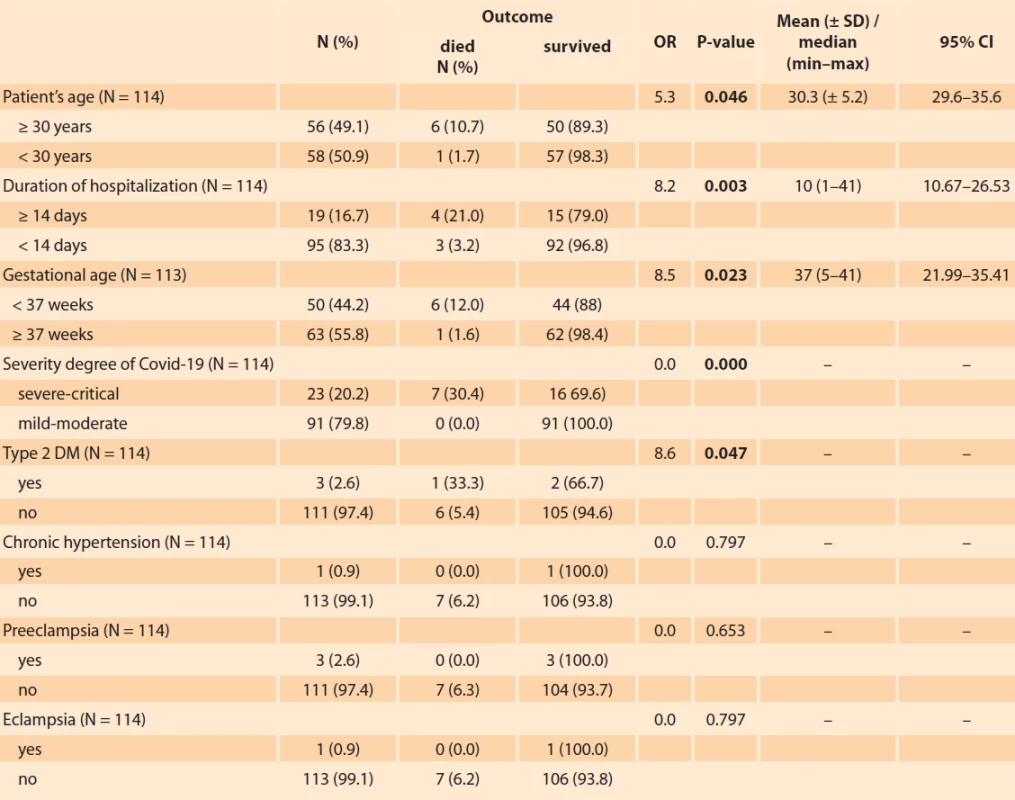

ACS – acute coronary syndrome, APTT – activated partial thromboplastin time, BGA – blood gas analysis, CAD – coronary artery disease,CHF – chronic heart failure, CI – confi dence interval, CRP – C-reactive protein, covid-19 – coronavirus disease 2019, DM – diabetes mellitus, eGFR – estimated glomerular fi ltration rate: procalcitonin, ESR – erythrocyte sedimentation rate, IL-6 – interleukin-6, max – maximum, min –minimum, N – number, OR – odds ratio, PT – prothrombin time, SD – standard deviation, SGOT – serum glutamic oxaloacetic transaminase, SGPT – serum glutamic pyruvic transaminase, UTI – urinary tract infection Discussion
The mortality in this study occurred in 7 (6.1%) patients. Different results were reported by Savasi et al and Zaigham et al, indicating no deaths in their study subjects [6,7].
Patient’s age
This study showed that pregnant women aged over or equal to 30 years was significantly related to a 5-fold increased risk of mortality (OR 5.3; P = 0.046). A meta - -analysis found that out of all the pregnant women who died, 41.7% were 35 years old, with a mortality risk of 0.61 times (P = 0.01; 95% CI 0.13–1.08) [8].
Duration of hospitalization
This study found that the length of hospitalization over or equal to 14 days was related to the increased risk of mortality up to 8 times (OR 8.2; P = 0.003). A study by Ayed et al showed 185 maternal patients infected by covid-19 had a median duration of treatment of 15 days (IQR 14–22) [9].
In this study, we didn’t analyse the relationship between the length of stay in ICU and maternal outcome, but several studies reported it. Karimi et al reported that maternal patients with covid-19 had a 5-fold higher ICU admission rate (RR: 5.04; 95% CI 3.13–8.10) than those without covid-19. The duration of hospitalization in ICU of patients with covid-19 and without covid-19 had a mean of 7 days and 2 days, respectively. Patients with covid-19 had a risk of 3.7-fold longer duration of stay in the ICU (P = 0.01; 95% CI 2.37–5.86) [8].
Gestational age
There was a significant relationship between gestational age and mortality rate in this study. Gestational age less than 37 weeks was significantly related to increased risk of mortality (OR 8.5; P = 0.023).
Karimi et al reported that covid-19 increased the risk of preterm birth (< 37 weeks) by almost 1.6-fold (RR 1.59; 95% CI 1.30–1.94). In addition, covid-19 patients with at least one comorbidity were found to have an increased risk of preterm birth (RR 1.96; 95% CI 1.41–2.73). Those comorbidities include diabetes, thyroid and endocrine disease, heart disease, hypertension, chronic lung disease, kidney disease, malaria, and tuberculosis [8].
Severity degree of covid-19
There was a significant relationship between the severity degree of covid-19 and mortality (P = 0.000). No patients died in the mild-moderate covid-19 group. Zhang et al also reported a significant relationship (P < 0.05) between severe-critical covid-19 and mortality, as indicated by univariate and multivariate regression. However, the subjects were not limited to only pregnant women [10].
Comorbidities
The comorbidities evaluated in this study were type 2 diabetes mellitus, chronic hypertension, preeclampsia, eclampsia, CHF, CAD/ACS, and asthma. The patients who died had type 2 diabetes mellitus, CHF, and CAD/ACS as their comorbidities. Meanwhile, no patients with chronic hypertension, preeclampsia, eclampsia or asthma died.
CHF had a 42-fold mortality risk (OR 42.4; P = 0.000), and CAD/ACS had an almost 18-fold mortality risk (OR 17.7; P = 0.009). These results were in accordance with the meta-analysis study conducted by Karimi-Zarchi et al. His study reported that there was no significant difference in the prevalence of preeclampsia between maternal patients with covid-19 compared to those without covid-19 infection (OR 1.676; P = 0.236) [11]. The meta-analysis by Karimi et al reported that out of the total number of patients who died, 9.1% of them had asthma, 31.1% had diabetes, and 14.1% had cardiovascular disease (CVD). The study reported that the risk of mortality was increased by 0.41-fold (P = 0.09) in patients with asthma, 0.29--fold (P = 0.12) in patients with diabetes mellitus, and 0.03-fold (P = 0.32) in patients with CVD [6]. Meta-analysis study by Rodriguez-Morales et al on covid-19 patients reported complications in the form of acute cardiac injury with a prevalence of 13% (95% CI 4.1–21.9; P = 0.035) out of a total 231 subjects [12].
Villar et al reported that pregnant women with covid-19 had a nearly 1.8--fold risk of developing preeclampsia/eclampsia/HELLP (RR 1.76; 95% CI 1.27–2.43). The study also compared the covid-19 group with at least one comorbidity (diabetes, thyroid and endocrine disease, heart disease, hypertension, chronic lung disease, kidney disease, malaria, and tuberculosis) and the covid-19 group without comorbidities. The study evaluated the outcome in the form of maternal morbidity and mortality index (MMMI) and its relationship with the presence of one of several complications, including vaginal bleeding, pregnancy-induced hypertension, preeclampsia, eclampsia, HELLP, preterm birth, infection, maternal death, ICU admission, and referral to a higher-level health facility. The occurrence of MMMI in the covid-19 group with comorbidities had a 1.7-fold risk (RR 1.71; 95% CI 1.33–2.20) [9].
It has not been determined whether SARS-CoV-2 infection causes an increased risk of preeclampsia. This undetermined causal condition was also supported by a study by Shanes et al, which reported that placental blood vessels in pregnant women infected with covid-19 underwent the same changes as in preeclampsia women [13]. In addition, the study conducted by Narang et al also demonstrated that systemic inflammatory and hypercoagulable conditions occur both in preeclampsia and non-pregnant covid-19 patients with chronic diseases [14].
Radiological image
The radiological image analysed in this study was the earliest examination taken at the time of admission. There was no significant relationship between abnormalities in chest X-rays and mortality rate (P = 0.582). This result was in accordance with the study of Rodriguez-Morales et al, which reported that unilateral pneumonia was present in 25.0% of subjects (P < 0.001), bilateral in 72.9% of subjects (P < 0.001), and ground-glass opacity in 68.5% of subjects (P < 0.001) [12]. Slightly different results were obtained from the study by Ayed et al, which reported that only 8 out of 52 patients (15.4%) had pneumonia from the chest X-ray results [9]. There was no study reporting the relationship between abnormalities in chest X-rays and mortality rate.
Hemoglobin
Hemoglobin levels analysed in this study was the earliest test taken at the time of admission. The median of hemoglobin levels in this study was 11.4 g/dL. There was no patient who died in the group with hemoglobin levels less than 11 g/dl. There was a significant relationship between hemoglobin levels and mortality (P = 0.043). A study by Dinevari et al on covid-19 patients reported that anemia significantly affected patients‘ mortality rate. The study reported that anemia increased mortality risk by nearly 1.7-fold (OR 1.68; P = 0.01) [15]. Another study also reported that pregnant women with gestational age more than 27 weeks and low hemoglobin levels (less than 10 g/dL) were related to increased mortality rate [4].
Leukocytes
Leukocyte count analysed in this study was the earliest test taken at the time of admission. The median of the leukocyte count was 9.100/µL. There was a significant relationship between the leukocyte count of ≥ 18.000/µL and mortality rate (OR 8.1; P = 0.011). A study conducted by Originkar et al reported the same result. Within 9 maternal patients with covid-19 who died, 5 patients had a leukocyte count greater than 18.000/µL [16]. A meta-analysis conducted by Shi et al reported that an increase in the leukocyte count was present in 33% of covid-19 patients (95% CI 0.11–0.60) [17]. Furthermore, a meta-analysis of 19 studies reported that leukopenia was present in 18.7% (95% CI 8.5–28.8; P < 0.001) of the total of 517 subjects, while leukocytosis was present in 16.8% (95% CI 5.5–28.0; P < 0.001) of the total of 487 subjects [12].
Lymphocytes
Lymphocyte levels analysed in this study was the earliest test taken at the time of admission. The result showed a significant increase in mortality risk in the presence of lymphocytopenia (OR 6.9; P = 0.045). Shi et al supported this result, which reported that a decrease in lymphocyte count was present in 59% of covid-19 patients (95% CI 0.41–0.75) [17]. A systematic review study by Zaigham et al also reported that 40 out of 68 cases (59%) had lymphocytopenia [7].
Lymphocytopenia is thought to be caused by these mechanisms: First, the virus infects lymphocytes directly, considering that lymphocytes also have angiotensin-converting enzyme 2 (ACE-2) receptors. Second, the virus attacks the body‘s lymphatic organs. Therefore, there will be an inflammatory cytokine imbalance such as IL-6 and tumor necrosis factor-alpha (TNF-a), causing apoptosis in lymphocytes. Finally, the presence of metabolic diseases may produce lactic acid resulting in inhibition of the lymphocyte proliferation process [18].
Neutrophils
Neutrophil levels analysed in this study was the earliest test taken at the time of admission. It was found that neutrophil levels over or equal to 80% had a significant relationship with an increase in mortality rate (OR 5.2; P = 0.036). A meta--analysis of 11 articles by Shi et al reported that the neutrophil level increase was present in 81% of covid-19 patients (95% CI 0.69–0.91) [17].
Neutrophils have a mechanism called neutrophil extracellular traps (NETs) against viral, bacterial, and fungal infection. The response occurs mainly in the respiratory tract. In this cellular response, neutrophils can lose part of the nucleus, which causes tissue damage. Viral infection may cause NETs, resulting in various manifestations such as alveolitis, endothelial damage, and intravascular coagulation [19].
Platelets
Platelet count analysed in this study was the earliest test taken at the time of admission. The mean of the platelet count in this study was 278,100/µL. Platelet count of ≤ 100,000/µL was significantly related to increased risk of mortality by 42 times (P = 0.000). Ayed et al, in their study of 185 maternal patients infected by covid-19, reported that the median of the platelet count was 214,000/µL (IQR 178,000–252,000) with only two patients who had a platelet count less than 100,000/µL (1.1%) [7].
Coagulation status
Coagulation test results analysed in this study were the earliest test taken at the time of admission. In this study, PT and APTT results were described as prolonged, shortened, or normal. There was no significant relationship between mortality rate and abnormalities in prothrombin time and activated partial thromboplastin time in this study. In the meta-analyses of 22 studies conducted by Jin et al on covid-19 infected patients, the results were not much different. The study compared severe/non-severe Covid group with prolonged prothrombin time (mean difference: 0.65 s; 95% CI 0.36–0.95; P < 0.05) and shortened activated partial thromboplastin time (mean difference: –0.01 s; 95% CI –2.58 to 2.56; P = 0.99). The mean of the prothrombin time was 12.20 s (95% CI 11.52–12.84) and the mean of activated partial thromboplastin time was 31.53 s (95% CI 28.46–34.60) [20].
D-dimer
D-dimer level analysed in this study was the earliest test taken at the time of admission. The mean of the D-dimer level in this study was 1,951.9 ng/mL. There was a significant relationship between mortality rate and D-dimer level over or equal to 2,500 ng/mL (OR 7.3; P = 0.028). This result is in accordance with a meta--analysis study by Shi et al, which reported that D-dimer level increased in 82% of cases (95% CI 0.75–0.89) [17]. The study of Ayed et al reported D-dimer levels increased in 100 patients (N = 185) with a median of 488.5 ng/mL (IQR 333–754.5). About 6% of the subjects had D-dimer levels of 500–1,000 ng/mL, and 4% of the subjects had D-dimer levels > 1,500 ng/mL [9].
Coagulation abnormality in covid-19 patients is a predictor of poor prognosis [21]. The coagulopathy abnormality in covid-19 infection begins with the process of endothelial damage. Laboratory indicators such as increased D-dimer, decreased platelets, prolonged prothrombin time, and decreased fibrinogen level can predict the presence of a coagulation disorder. In the early stage of covid-19, it was expected to find elevated platelets, fibrinogen, von Willebrand factor (VWF), and P-selectin; however, the D-dimer level may be normal or increased. In the next stage, the D-dimer level rose rapidly, platelet count increased, while the fibrinogen, VWF and P-selectin levels were still high. This condition may cause hypercoagulation and thrombosis. In critically ill patients, the level of D-dimer and P-selectin is higher. However, platelet count, fibrinogen level, and VWF level will decrease due to a reduced amount in the circulation due to coagulopathy conditions or continuous endothelial cell damage [22].
Erythrocyte sedimentation rate
Erythrocyte sedimentation rate over 100 mm showed a significant relationship with an increase in mortality risk (OR 15.0; P = 0.003). A meta-analysis of 11 articles by Shi et al reported that pregnant women with covid-19 had an increased erythrocyte sedimentation rate in 29% of cases (95% CI 0.20–0.39) [17]. Another study also found an increase in the erythrocyte sedimentation rate with a prevalence of 41.8% (95% CI 0.0–92.8; P < 0.001) in 157 subjects [12].
C-reactive protein
C-reactive protein levels analysed in this study was the earliest test taken at the time of admission. In covid-19 infection, CRP can be an indicator for a cytokine storm or secondary bacterial infection [21]. The cut-off of the CRP value used in this study was 32 mg/L. Shi L et al, in their meta-analysis, reported that the CRP value increased in 69% of cases of covid-19 (95% CI 0.58–0.79) [17]. A systematic review by Zaigham et al also reported that 45 out of 64 cases (70%) had an elevated CRP [9]. Furthermore, a study of 332 subjects found that the percentage of elevated CRP was 58.3% (95% CI 21.8–94.7; P < 0.001) [12]. The high prevalence of an elevated CRP level in covid-19 infections made it important to find a new cut-off value to evaluate prognosis.
Interleukin-6
Interleukin-6 levels analysed in this study was the earliest test taken at the time of admission. In this study, the IL-6 value was found in only 16 out of 114 patients. There was no significant relationship between IL-6 level > 30 pg/mL and mortality rate (P = 0.515). A study by Tanacan et al reported that IL-6 had a significant relationship with CRP levels and disease severity, with P < 0.001 in both [23]. Hence, we hypothesize that it will be significantly related to mortality rate.
IL-6 is a cytokine that increases during pregnancy. IL-6 functions in pregnancy tolerance, especially during implantation and placental development. IL-6 imbalance can cause various complications such as preeclampsia, miscarriage, and preterm birth [24].
Transaminase enzymes
Transaminase enzyme levels analysed in this study was the earliest test taken at the time of admission. It was found that there was a significant relationship between transaminase enzyme levels and mortality rate. SGOT levels of ≥ 34 IU/L were related to increased risk of mortality rate (OR 11.7; P = 0.007). Meanwhile, SGPT levels of ≥ 55 IU/L were related to increased risk of mortality rate (OR 8.8; P = 0.005). Similar results were reported by previous studies. Research by Ayed et al (185 covid-19 patients, obtained SGOT data on 125 subjects and SGPT on 149 subjects) reported that the median of the SGOT level was 21 IU/L (IQR 16–28), with SGOT levels > 40 IU/L in 14 patients (11.2%). The median of the SGPT level was 13 IU/L (IQR 11–24), with SGPT levels > 40 IU/L in 36 patients (24.2%). A meta-analysis study reported a significant increase of SGOT and SGPT levels in covid-19 patients. The increase of SGOT levels was found in 33.3% of 169 subjects (95% CI 26.3–40.4). SGPT levels increased 24.1% in 128 subjects (95% CI 13.5–34.6) [24].
Serum electrolytes
Serum electrolyte levels analysed in this study was the earliest test taken at the time of admission. Serum electrolytes evaluated in this study were sodium, potassium, and chloride. Sodium and potassium levels were significantly related to the mortality rate of covid-19 infected mothers (OR 6.1; P = 0.018) and (OR 10.7; P = 0.003), but chloride levels were not significantly related to mortality rate. Sodium levels < 135 mEq/L were related to increased mortality risk. Lippi et al reported results that were not much different. They reported that sodium levels had a significant relationship with mortality, while potassium and chloride did not.
Some conditions, including a mild degree of covid-19, fever and lack of fluid intake, can cause electrolyte imbalances. Dehydration may cause disturbances in kidney function, as indicated by the presence of acute kidney injury [25].
Kidney function
Kidney function results analysed in this study was the earliest test taken at the time of admission. Kidney function laboratory indicators, such as urea, creatinine, and eGFR, significantly related to mortality rate. Urea levels ≥ 50 mg/dL were related to increased mortality risk by 11-fold (OR 11.1; P = 0.005). Creatinine levels ≥ 0.75 mg/dL were related to increased mortality risk by 8-fold (OR 8.2; P = 0.004). Meanwhile, eGFR < 90 mL/min/1.73 m2 was significantly related to increased mortality risk by almost 9-fold (OR 8.5; P = 0.004). A meta-analysis study of 36 studies in covid-19 patients reported a significant increase in urea and creatinine, and a decrease in eGFR in covid-19 patients. In addition, complications related to acute kidney injury also increased significantly [26]. Rodriguez-Morales et al reported that the prevalence of acute kidney injury in 330 covid-19 patients was 7.9% (95% CI 1.8–14.0; P < 0.001) [12].
As explained previously, acute kidney injury can be caused by dehydration. Severe dehydration may lead to shock, resulting in acute tubular necrosis. A direct attack by the virus on the ACE-2 receptor in kidneys is almost 100 times more likely than it is in the respiratory system. Finally, the presence of other comorbidities, such as hypertension and diabetes, may also increase the risk of acute kidney injury [27].
Procalcitonin
Procalcitonin levels analysed in this study was the earliest test taken at the time of admission. It was found that the procalcitonin value was 0.5 ng/mL with a mean value of 0.78 ng/mL, and the median value was 0.20 ng/mL. The value was related to increased mortality risk (OR = 5.7; P = 0.043). Research conducted by Hu et al reported that the increase in procalcitonin was in accordance to the severity of covid-19. Compared to the clinically moderate group, the clinically severe group had a 4-fold increase in procalcitonin, while the critical ill group had an 8-fold increase in procalcitonin [28].
Several other studies reported different results regarding the median procalcitonin value. Research by Ayed et al evaluated the procalcitonin values of pregnant women infected by covid-19. The median procalcitonin value was 0.05 ng/mL (IQR = 0.05-0.08) and the number of patients with procalcitonin levels > 0.2 ng/mL was 12 (11.1%) [9]. Another study by Yang et al reported that the median procalcitonin value was 0.06 ng/mL in covid-19 patients (IQR = 0.05–0.16) [29].
Blood gas analysis
Blood gas analysis results analysed in this study was the earliest test taken at the time of admission. This study grouped the research subjects into normal and abnormal (acidosis/alkalosis) blood gas analysis results. No significant relationship was found between abnormalities in the blood gas analysis and mortality rate. This result was different compared to the research by Anggara et al, which reported that 80.51% out of 205 subjects had abnormal blood gas analysis results. The study also reported a significant relationship between mortality rate and several components of the blood gas analysis (P < 0.05) [30]. Another study by Kleninger et al using a multivariate analysis reported that the abnormalities in the pH value affected mortality rate [31].
Urinary tract infections
Urinary test examination analysed in this study was the earliest test taken at the time of admission. Urinary tract infection was significantly related to mortality rate (OR = 21.9; P = 0.001). This result was in accordance to some previous studies. Haocheng Zhang et al reported that 22 out of 38 subjects (57.9%) had secondary infections, one of which was a UTI. The study also reported that the critically ill group had a significantly greater secondary infection risk than the clinically moderate group (P < 0.0001) [32]. Another study by Marand et al reported a significant relationship between mortality rate and urinary leukocytes (P < 0.005). These results can be due to the virus attacking the ACE-2 receptors in the bladder [33].
Antibiotics
In this study, antibiotics were administered mainly as prophylaxis before surgery and as therapeutic therapy for secondary infections. We did not differentiate the purpose of antibiotic administration. Antibiotics used were ceftriaxone, cefazoline, meropenem and tegacycline.
There was no significant relationship between maternal mortality and antibiotic therapy (P = 0.738). Different results were reported by Bendala Estrada et al in their study which evaluated the use of three types of antibiotics in covid-19 patients. It was reported that antibiotic therapy significantly increased the risk of mortality 1.4 times (OR % 1.39; P < 0.001). Different results were reported in the group receiving macrolide antibiotics, which showed a better survival rate (OR = 0.70; P < 0.001) [34]. Another study by Buetti et al reported that the mortality rate between the groups with and without antibiotics was not significantly different at 26.3% and 24.3% (P = 0.86) respectively [35].
Antivirals
Antiviral therapy was given to 66.7% of all subjects in this study. The antiviral regimens given were favipiravir, remdesivir, and oseltamivir. There were no subjects who died in the group without antivirals. The result was reasonable considering that the cases without antiviral therapy were covid-19 cases with mild symptoms.
The result of this study indicated that there was no significant relationship between the use of antivirals and mortality rate. The study conducted by Burwick et al in 86 pregnant women reported that the administration of remdesivir resulted in good outcomes. In the group in which remdesivir was given the first-time they were diagnosed with covid-19 infection during pregnancy, 93% had improved clinical conditions. In the group in which remdesivir was administered after termination, 90% had improved conditions [36]. Another study by Kim et al reported a significant relationship between the use of remdesivir and mortality rate (P = 0.003). In addition, remdesivir was also found to be significantly associated with an increase in the severity of covid-19 symptoms (P = < 0.001) [37].
Steroids
In this study, steroid regimens given were dexamethasone or methylprednisolone. The use of dexamethasone in this study was mostly aimed for fetal lung maturation, while methylprednisolone served as a covid-19 therapeutic agent. The use of steroids showed a significant relationship with mortality rate (P = 0.006). Specific studies regarding maternal mortality of covid-19 and its correlation with steroid use have not been found. The study of covid-19 patients in the general population by Kim et al reported that there was a significant relationship between steroid use and decreased mortality in ICU patients (P < 0.001), non-ICU patients (P = 0.002), and disease severity progression (P < 0.001) [37].
Tocilizumab
This study shows that the administration of tocilizumab increased the risk of mortality (OR = 21.0; P = 0.001). Due to the development of science and updates on covid-19 therapy, tocilizumab was not widely used at the time this study was conducted. Tocilizumab in this study was only given to 4 subjects (3.5%). It is estimated that this significant result occurred due to the limitation of the number of subjects and the consideration of tocilizumab in certain severe cases. A study reported different results, where tocilizumab significantly reduced the risk of mortality rate in the ICU (P = 0.012), non-ICU (P < 0.001), and prevention groups (P < 0.001) [37]. Furthermore, Jorgensen et al suggested that tocilizumab did not show any serious side effects on pregnancy [38]. However, a larger number of subjects is needed to evaluate this relationship further.
Pregnancy termination
Termination of pregnancy in this study was performed in 86 subjects (78%), with caesarean section in 74 subjects (86%). The termination of pregnancy was indicated by obstetrical indication, fetal distress, respiratory failure, or covid-19 infection at term pregnancy. In our center, we prevent covid-19 spread to health providers by performing caesarean section in covid-19 infected pregnant women at term pregnancy. Those decisions were also made considering a worse prognosis in pregnant women infected by covid-19 since the Delta variant emerged. Vaginal deliveries were performed in patients who were in labour at admission.
In this study, there was no significant relationship between pregnancy termination and mortality rate (P = 0.508). This result was not different from several previous studies. Savasi et al, in their study on 77 pregnant women, reported that 57 (74%) patients required termination, while 20 (26%) patients had improved. Of these 57 patients, 35 (61%) patients were terminated by caesarean section, and 22 (39%) by vaginal deliveries [6]. The study conducted by Osaikhuwuomwan et al reported that 11 (57.9%) out of 67 patients had a termination. Five (45.4%) people terminated the pregnancy vaginally, and six (54.6%) others did caesarean section [39]. A systematic review by Zaigham et al of 108 pregnant women infected by covid-19 reported that 101 (92%) patients had caesarean section, while seven (8%) patients had vaginal delivery. Various studies stated that the indication for cesarean section was mainly due to fetal distress. However, there was no detailed explanation regarding the condition, whether from abnormal cardiotocography (CTG), amniotic fluid, or fetal acidosis [7].
Research by Villar et al reported that the risk of preterm birth (< 37 weeks) increased almost 1.6-fold in covid-19 patients (RR = 1.59; 95% CI 1.30–1.94). The type of termination of pregnancy in the form of cesarean section in covid-19 patients increased almost 1.3 times (RR = 1.28; 95% CI 1.16–1.40). At the time of termination, covid-19 patients had a mean gestational age of 37 weeks, and the group without covid-19 had a mean gestational age of 38 weeks (RR = –0.61; 95% CI –0.90 to –0.32) [4].
Conclusion
Various factors were significantly related to the mortality rate of pregnant women with covid-19. These factors could be derived from the patient‘s baseline data obtained from the history, investigations, and interventions during treatment. This study may become the basis in monitoring patients‘ conditions, thus hopefully reducing the risk of mortality in pregnant women with covid-19. The results also showed that there was a possibility to reassess the effectivity of the laboratory examination and therapy performed in covid-19 patients. Several supporting examinations that were found to have no correlation with maternal mortality can be abandoned under conditions of limited resources. Limitations in this study include incomplete subject data and a small number of pregnant women died. Further research is needed to evaluate the correlation of these factors with maternal mortality in a larger number of subjects.
Submitted/Doručeno: 15. 1. 2023
Accepted/Přijato: 11. 5. 2023Siti Azizah, MD
Universitas Indonesia Hospital
Pondok Cina 16424
Depok, Indonesia
siti.azizah.obg@gmail.com
Sources
1. World Health Organization. Covid-19 Weekly Epidemiological Update 22. Geneva 2021 [online]. Available from: https: //www.who.int/docs/defaultsource/coronaviruse/situation-reports/weekly_epidemiological_update_22.pdf.
2. Ophinni Y, Hasibuan AS, Widhani A et al. Covid-19 vaccines: current status and implication for use in indonesia. Acta Med Indones 2020; 52 (4): 388–412.
3. Perkumpulan Obestetri dan Ginekologi Indonesia. Covid-19 2021 [online]. Available from: https: //pogi.or.id/publish/category/covid-19 /.
4. Villar J, Ariff S, Gunier RB et al. Maternal and neonatal morbidity and mortality among Pregnant women with and without Covid-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021; 175 (8): 817–826. doi: 10.1001/jamapediatrics.2021.1050.
5. Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 2012; 62 (3): 263–271. doi: 10.1016/j.yhbeh.2012.02.023.
6. Savasi VM, Parisi F, Patanè L et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (Covid-19). Obstet Gynecol 2020; 136 (2): 252–258. doi: 10.1097/AOG.0000000000003979.
7. Zaigham M, Andersson O. Maternal and perinatal outcomes with Covid-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020; 99 (7): 823–829. doi: 10.1111/aogs.13867.
8. Karimi L, Makvandi S, Vahedian-Azimi A et al. Effect of Covid-19 on mortality of pregnant and postpartum women: a systematic review and meta-analysis. J Pregnancy 2021; 2021 : 8870129. doi: 10.1155/2021/8870129.
9. Ayed A, Embaireeg A, Benawadh A et al. Maternal and perinatal characteristics and outcomes of pregnancies complicated with Covid-19 in Kuwait. BMC Pregnancy Childbirth 2020; 20 (1): 754. doi: 10.1186/s12884-020-03461-2.
10. Zhang J, Wang X, Jia X et al. Risk factors for disease severity, unimprovement, and mortality in Covid-19 patients in Wuhan, China. Clin Microbiol Infect 2020; 26 (6): 767–772. doi: 10.1016/j.cmi.2020.04.012.
11. Karimi-Zarchi M, Schwartz DA, Bahrami R et al. A meta-analysis for the risk and prevalence of preeclampsia among pregnant women with Covid-19. Turk J Obstet Gynecol 2021; 18 (3): 224–235. doi: 10.4274/tjod.galenos.2021.66750.
12. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E et al. Clinical, laboratory and imaging features of Covid-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 34 : 101623. doi: 10.1016/j.tmaid.2020.101623.
13. Shanes ED, Mithal LB, Otero S et al. Placental pathology in Covid-19. Am J Clin Pathol 2020; 154 (1): 23–32. doi: 10.1093/ajcp/aqaa089.
14. Narang K, Enninga EA, Gunaratne MD et al. SARS-CoV-2 infection and Covid-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc 2020; 95 (8): 1750–1765. doi: 10.1016/j.mayocp.2020.05.011.
15. Dinevari MF, Somi MH, Majd ES et al. Anemia predicts poor outcomes of Covid-19 in hospitalized patients: a prospective study in Iran. BMC Infect Dis 2021; 21 (1): 170. doi: 10.1186/s128 79-021-05868-4.
16. Asalkar M, Thakkarwad S, Rumani I et al. Prevalence of maternal mortality and clinical course of maternal deaths in Covid-19 pneumonia-A cross-sectional study. J Obstet Gynaecol India 2022; 72 (3): 208–217. doi: 10.1007/s132 24-021-01545-3.
17. Shi L, Wang Y, Yang H et al. Laboratory abnormalities in pregnant women with novel coronavirus disease 2019. Am J Perinatol 2020; 37 (10): 1070–1073. doi: 10.1055/s-0040-1712181.
18. Tan L, Wang Q, Zhang D et al. Lymphopenia predicts disease severity of Covid-19: a descriptive and predictive. Signal Transduct Target Ther 2020; 5 (1): 33. doi: 10.1038/s41392-020-0148-4.
19. Makatsariya A, Slukhanchuk E, Bitsadze V et al. Covid-19, neutrophil extracellular traps and vascular complications in obstetric practice. J Perinat Med 2020; 48 (9): 985–994. doi: 10.1515/jpm-2020-0280.
20. Jin S, Jin Y, Xu B et al. Prevalence and impact of coagulation dysfunction in Covid-19 in China: a meta-analysis. Thromb Haemost 2020; 120 (11): 1524–1535. doi: 10.1055/s-0040-1714369.
21. Al-Saadi EA, Abdulnabi MA. Hematological changes associated with Covid-19 infection. J Clin Lab Anal 2022; 36 (1): e24064. Doi: 10.1002/jcla.24064.
22. Grobler C, Maphumulo SC, Grobbelaar LM et al. Covid-19: the Rollercoaster of Fibrin (Ogen), D-Dimer, von Willebrand factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int J Mol Sci 2020; 21 (14): 5168. doi: 10.3390/ijms21145168.
23. Tanacan A, Yazihan N, Erol SA et al. The impact of Covid-19 infection on the cytokine profile of pregnant women: a prospective case-control study. Cytokine 2021; 140 : 155431. doi: 10.1016/j.cyto.2021.155431.
24. Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol 2012; 95 (1–2): 1–14. doi: 10.1016/j.jri.2012.05.004.
25. Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (Covid-19). Ann Clin Biochem 2020; 57 (3): 262–265. doi: 10.1177/00 04563220922255.
26. Liu YF, Zhang Z, Pan XL et al. The chronic kidney disease and acute kidney injury involvement in Covid-19 pandemic: a systematic review and meta-analysis. PLoS One 2021; 16 (1): e0244779. doi: 10.1371/journal.pone.0244779.
27. Valizadeh R, Baradaran A, Mirzazadeh A et al. Coronavirus-nephropathy; renal involvement in Covid-19. J Renal Inj Prev. 2020; 9 (2): e18. doi: 10.34172/jrip.2020.18.
28. Hu R, Han C, Pei S et al. Procalcitonin levels in Covid-19 patients. Int J Antimicrob Agents 2020; 56 (2): 106051. doi: 10.1016/ j.ijantimicag.2020.106051.
29. Yang H, Hu B, Zhan S et al. Effects of severe acute respiratory syndrome coronavirus 2 infection on pregnant women and their infants. Arch Pathol Lab Med 2020; 144 (10): 1217–1222. doi: 10.5858/arpa.2020-0232-SA.
30. Anggara B. Penilaian Analisis Gas Darah dan AaDO2 pada saat pertama kali masuk sebagai faktor prognostik pada pasien Covid-19 terkonfirmasi yang meninggal di RSUP Persahabatan = Arterial blood gas analysis and alveolar-arterial oxygen gradient as predictors of mortality in Covid-19 confirmed patients treated at Persahabatan Hospital, Jakarta. Jakarta: Program Studi Pulmonologi & Ilmu Kedokteran Respirasi. 2021 [online]. Available from: https: //perpustakaan.fk.ui.ac.id/new-opac/index.php?p=show_ detail&id=27242&keywords=.
31. Kieninger M, Sinning A, Vadász T et al. Lower blood pH as a strong prognostic factor for fatal outcomes in critically ill Covid-19 patients at an intensive care unit: a multivariable analysis. PLoS One 2021; 16 (9): e0258018. Doi: 10.1371/journal.pone.0258018.
32. Zhang H, Zhang Y, Wu J et al. Risks and features of secondary infections in severe and critical ill Covid-19 patients. Emerg Microbes Infect 2020; 9 (1): 1958–1964. doi: 10.1080/222 21751.2020.1812437.
33. Marand AJ, Bach C, Janssen D et al. Lower urinary tract signs and symptoms in patients with Covid-19. BMC Infect Dis 2021; 21 (1): 706. doi: 10.1186/s12879-021-06394-z.
34. Estrada AD, Parra JC, Carracedo EF et al. Inadequate use of antibiotics in the covid-19 era: effectiveness of antibiotic therapy. BMC Infect Dis 2021; 21 (1): 1144. doi: 10.1186/s12 879-021-06821-1.
35. Buetti N, Mazzuchelli T, Lo Priore E et al. Early administered antibiotics do not impact mortality in critically ill patients with Covid-19. J Infect 2020; 81 (2): e148–e149. Doi: 10.1016/ j.jinf.2020.06.004.
36. Burwick RM, Yawetz S, Stephenson KE et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis 2021; 73 (11): e3996–e4004. Doi: 10.1093/cid/ciaa1466.
37. Kim MS, An MH, Kim WJ et al. Comparative efficacy and safety of pharmacological interventions for the treatment of Covid-19: a systematic review and network meta-analysis. PLoS Med 2020; 17 (12): e1003501. doi: 10.1371/journal.pmed.1003501.
38. Jorgensen SC, Lapinsky SE. Tocilizumab for coronavirus disease 2019 in pregnancy and lactation: a narrative review. Clin Microbiol Infect 2022; 28 (1): 51–57. doi: 10.1016/j.cmi. 2021.08.016.
39. Osaikhuwuomwan J, Ezeanochie M, Uwagboe C et al. Clinical characteristics and outcomes for pregnant women diagnosed with Covid-19 disease at the University of Benin Teaching Hospital, Benin City, Nigeria. Pan Afr Med J 2021; 39 : 134. doi: 10.11604/pamj.2021.39.134.27627.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2023 Issue 5-
All articles in this issue
- Morbidly adherent placenta as a cause of peripartum hysterectomy in the Slovak Republic in the years 2012–2020
- Maternal and fetal outcomes in subsequent pregnancies of patients who underwent Acar-style conservative surgery for placenta accreata spectrum
- Factors related to maternal mortality rate in covid-19 patients – a cross-sectional study from an Indonesian covid-19 referral hospital
- Implementation of the ERAS protocol in gynecology and oncogynecology – evaluation of a pilot study
- Monitoring the relationship between overactive bladder and mobility disorders in women with multiple sclerosis
- Relugolix combination therapy and symptoms of uterine myomatosis – selected case reports of indication spectrum and treatment outcomes
- Nascent myoma as a cause of urinary retention
- Diagnosis of thanatophoric dysplasia using clinical exome screening
- Viable invasive cervical pregnancy treated with minimally invasive procedures
- Early treatment of vulvar synechiae in childhood – prevention of late complications
- Home births as a "right" of female patients in the context of medicine, legislation and court jurisprudence
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Early treatment of vulvar synechiae in childhood – prevention of late complications
- Relugolix combination therapy and symptoms of uterine myomatosis – selected case reports of indication spectrum and treatment outcomes
- Implementation of the ERAS protocol in gynecology and oncogynecology – evaluation of a pilot study
- Nascent myoma as a cause of urinary retention
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career



