-
Medical journals
- Career
Intestinal metaplasia of the stomach and esophagus: an immunohistochemical study of 60 cases including comparison with normal and inflamed intestinal mucosa
Authors: Alena Chlumská 1,2; Petr Mukenšnabl 1; Petr Mareček 3; Michal Zámečník 4,5
Authors‘ workplace: Šikl's Department of Pathology, Faculty Hospital in Pilsen, Charles University in Prague, Pilsen, Czech Republic 1; Laboratory of Surgical Pathology, s. r. o., Pilsen, Czech Republic 2; Medicentrum, s. r. o., Beroun, Czech Republic 3; Department of Pathology, AGEL Laboratories, a. s., Nový Jičín, Czech Republic 4; Medicyt, s. r. o., Laboratory Trenčín, Trenčín, Slovak Republic 5
Published in: Čes.-slov. Patol., 50, 2014, No. 3, p. 141-148
Category: Original Article
Overview
Recently, a new classification of intestinal metaplasia (IM) using immunohistochemical mucin markers was proposed. Two following types of IM were defined: (1) a mixed gastric and intestinal type also called incomplete IM; (2) a purely intestinal type, also called complete IM. We present a series of 30 cases of gastric IM and 30 cases of IM of the esophagus, using this new classification.
In all gastric cases, IM developed in the mucus-neck region in the form of incomplete IM. Toward the mucosa surface, it matured gradually into complete IM. This maturation showed a gradual reduction of both foveolar mucin MUC5AC and pyloric gland mucin MUC6. In two of 30 cases, IM was of the incomplete hyperproliferative type. In one case, focal high-grade adenomatous dysplasia was found in the incomplete IM.
In the esophageal cases, IM was found in inflamed cardiac-type mucosa, and it was usually of the incomplete type, with almost diffuse positivity for MUC5AC and with rare positivity of MUC6. The goblet cells and some cylindrical cells expressed intestinal mucin MUC2. The proliferation was higher than in the complete IM, and in one case, focal low grade adenomatous dysplasia was found.
In addition, we examined the expression of mucins in normal and inflamed intestinal mucosa. These cases included 50 duodenal biopsies, 50 biopsies from the ileum, and 50 biopsies from the colon. The inflamed cases included celiac disease, Crohn's disease, and ulcerative colitis. Some goblet cells of the normal intestinal mucosa expressed both MUC2 and MUC5AC. More numerous MUC5AC+ goblet cells were found in the inflamed intestinal mucosa. In the duodenal and small intestinal mucosa, even the MUC6 positivity of a few goblet or cylindrical cells was found.
In sum, our results indicate that incomplete IM is an initial step of the metaplastic process. It can mature into complete IM, or alternatively, it can develop dysplasia or adenocarcinoma. In addition, we found that gastric-type mucins are also present in normal or inflamed intestinal mucosa, and that the expression of these mucins is even enhanced in some inflammatory conditions. The expression of MUC5AC in complete IM and in normal or inflamed mucosa suggests that MUC5AC cannot be regarded as a marker of immaturity. In Barrett esophagus, our results were similar to those of previous studies, except for CDX2 of which reactivity was seen also in incomplete type of IM.Keywords:
intestinal metaplasia – immunohistochemical classification – intestinal mucin – gastric mucins – Barrett esophagusIntestinal metaplasia (IM) is defined as the replacement of the native mucosa by an epithelium resembling the small bowel mucosa (1,2). IM has been classically divided into type I (small intestinal mucosa), type II (incomplete) and type III (colonic) on the basis of morphology and intestinal mucin histochemistry (expression of sialomucins and sulphomucins) (1,3). Using newly developed antibodies, following immunohistochemical classification of IM was proposed: 1) a mixed gastric and intestinal type, i.e. incomplete IM with features of both intestinal and gastric mucosa, generally lacking an absorptive epithelium, and 2) a purely intestinal type, i.e. complete IM with the presence of goblet cells, absorptive cells, neuroendocrine cells and Paneth cells (1,3-10). The absence of gastric mucins in complete IM (8) is interesting since it challenges the opinion that incomplete IM arises from a complete type. Recently, it has been suggested that incomplete IM (producing gastric mucins) represents an initial immature and unstable stage that may either undergo maturation into complete IM or which persists and progresses to dysplasia and carcinoma (3,4,6,8). In the stomach, the complete IM is the most common type. It is more frequently observed in the antral mucosa and its incidence increases with age (3,11). The pathogenesis of gastric IM is still largely unknown. At present, Helicobacter pylori (HP) infection has been established to be a major cause of chronic inflammation, and this inflammation can cause development of IM (3). Another condition with IM is autoimmune gastritis, in which IM occurs often together with so-called pseudopyloric metaplasia (1,12). In the esophagus, IM arises in a cardia-type mucosa secondary to chronic gastroesophageal reflux disease (13-15), and it is almost always incomplete (16). IM in the columnar lined esophagus has long been recognized as the most significant risk factor for esophageal dysplasia and adenocarcinoma (4,13,17).
We studied a series of IM in both gastric and esophageal biopsy specimens. We have applied a new IM classification (1,3,8), which uses immunohistochemistry for proof of intestinal - and gastric-types of mucin (MUC2, MUC5AC, MUC6), and we compared our results with those of previous studies. By these examinations, we found expression of gastric mucin MUC5AC also in quite mature-appearing goblet cells of the complete IM, and this finding prompted us to perform an immunohistochemical study of these mucins in the normal and inflamed mucosa of the intestine.
MATERIAL AND METHODS
Our study included antral gastric specimens with IM from 30 patients (13 male and 17 female, mean age 62 years, age range 33 - 87 years), and 30 distal esophageal biopsies with histologically confirmed Barrett esophagus (21 male and 9 female, mean age 51 years, age range 42 - 58 years). In addition, normal and inflamed mucosa of the duodenum, terminal ileum and colon was examined for expression of mucins. The duodenal group included 50 endoscopic biopsies (12 male and 38 female, mean age 42 years, age range 21 - 81 years). 39 of these biopsies showed normal duodenal mucosa and 11 of them had features of celiac disease (Marsh I – IIIC). The small intestinal control group included 50 specimens from the terminal ileum (29 male and 21 female, mean age 45 years, age range 18 - 82 years). Normal mucosa was found in 46 cases, and 4 cases showed Crohn’s disease. The colonic control group contained 50 biopsies of the colon from various parts of the colon (33 male and 17 female, mean age 59 years, age range 19 - 79 years). In 40 cases, the mucosa was normal, whereas 10 cases showed features of ulcerative colitis with mild activity.
All biopsy specimens were processed routinely and stained with hematoxylin-eosin and alcian blue/PAS stain (alcian blue at pH 2.5, periodic acid-Schiff reagent). In addition, gastric and esophageal biopsies were stained with silver impregnation of Helicobacter pylori. Immunohistochemistry was performed using a standard avidin-biotin complex peroxidase technique, and it included the following antibodies: MUC5AC (clone MRQ-19, Cell Marque), MUC6 (clone MRQ-20, Cell Marque), MUC2 (clone MRQ-18, Cell Marque), CD10 (clone SP67, Ventana), CDX2 (clone EPR2764Y, Cell Marque), p53 (clone D07, Ventana), AMACR (clone 13H4, DAKO) and Ki-67 (MIB1, Ventana). Gastric and esophageal biopsies were examined with all above-mentioned antibodies. The specimens from the duodenum, ileum and colon were examined with MUC5AC and MUC6. The classification criteria for gastric IM were as follows: complete (purely intestinal) IM showed MUC2+ goblet cells, continuous CD10+ brush border of the cylindrical absorptive cells, nuclear expression of CDX2, and lack of MUC5AC and MUC6. Incomplete IM showed expression of MUC5AC and MUC6 in the goblet cells, positivity of MUC5AC in the cylindrical cells, lack of the brush border (with negativity of CD10), MUC2 positivity in both goblet and cylindrical cells, and nuclear expression of CDX2 in both goblet and cylindrical cells (1,5-8,11).
RESULTS
The gastric IM was always antral. It developed in the mucus-neck region in the form of incomplete gastrointestinal type IM (Figs. 1A, B). Both goblet and cylindrical cells were positive for MUC5AC. Very rare goblet cells showed MUC6 expression (Fig. 1C). The goblet cells and very rare cylindrical cells were positive for MUC2. Some of the cylindrical cells had superficial brush border. Cell proliferation activity of the metaplastic epithelium (measured by MIB1 expression) was increased in the mucus-neck region and in the adjacent foveolas (Fig. 1D). Toward the mucosal surface, the metaplastic epithelium revealed a gradual reduction of MUC5AC in both cell types and a reduction of MUC6 in the goblet cells. In two cases, focal hyperproliferative incomplete IM was found in the foveolas (Figs. 2A, B). It showed closely packed glands lined by cells with mild irregular and basally located nuclei. Cell proliferation activity of the hyperproliferative IM was increased diffusely (Fig. 2C), and the nuclei of some cells were slightly p53 positive (Fig. 2D). AMACR was negative. In the superficial portion of the foveolas and in the superficial epithelium, IM was negative for MUC5AC and MUC6. In the complete IM (Fig. 3A), the cylindrical cells were of the mature-appearing absorptive type, and very rare Paneth cells were found. In only 10 cases (33 %) of the complete IM, the goblet cells showed persistence of MUC5AC positivity (Fig. 3B). Foci of incomplete IM in the superficial epithelium (in addition to complete IM) were found in two additional cases. They showed expression of MUC5AC in both goblet and cylindrical cells, and increased cell proliferation. In one 78-year-old woman, focal high grade dysplasia of the adenomatous type was found in the incomplete IM. This dysplasia was positive for CDX2 (Fig. 4A), it showed high cell proliferation, and it was negative for MUC5AC (Fig. 4B) and MUC6. The antral mucosa near the IM was normal in 15 cases, and it showed mild chronic inactive gastritis in 13 cases, of which two cases were HP positive. In 2 additional cases, HP positive chronic gastritis with mild activity was found (Table 1).
Fig. 1. Gastric incomplete intestinal metaplasia. A: intestinal epithelium-like features seen on HE stained section, B: alcian blue/PAS stain demonstrates production of mucins, C: MUC6 in rare goblet cells, D: MIB1positivity is increased in the mucus neck region (A: hematoxylin and eosin, magnification x100; B. alcian blue/PAS, magnification x 100; C and D: ABC technique, magnifications x100). 
Fig. 2. Gastric incomplete hyperproliferative IM. A: highly proliferative cellular epithelium, B: alcian blue/PAS stain shows mucin positivity, C: increased MIB1 positivity, D: expression of p53 (A: hematoxylin and eosin, magnification x200; B: alcian blue/PAS, magnification x200; C and D: ABC technique, magnifications x100). 
Fig. 3. Gastric complete intestinal metaplasia. A: alcian blue/PAS stain shows a well developed PAS positive brush border and alcian blue positive goblet cells, B: persistence of MUC5AC as it was seen in 33% of cases (A: alcian blue/PAS, magnification x 100; B: ABC technique, magnification x100). 
Fig. 4. Gastric incomplete intestinal metaplasia with high grade dysplasia of the adenomatous type. A: positivity for CDX2, B: absence of MUC5AC is limited to the dysplastic glands (ABC technique, magnifications x100). 
1. The findings in the cases of IM of the stomach. 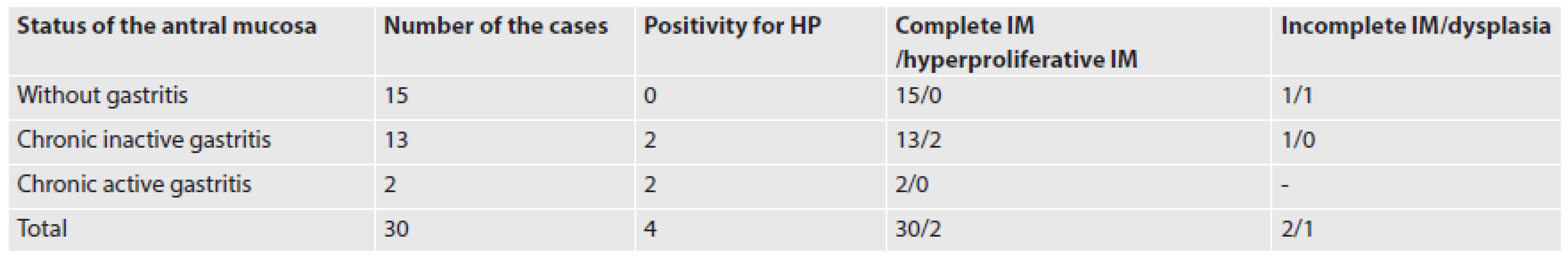
HP: Helicobacter pylori; IM: intestinal metaplasia In the esophagus, IM was seen in the cardia-type mucosa which was covered partially by the squamous epithelium. This IM was of the incomplete type in all 30 cases (Figs. 5A, B). However, in 3 cases, very small foci of complete IM were found on the mucosal surface. The incomplete IM contained less well-differentiated goblet and cylindrical cells, and, exceptionally, Paneth cells. A majority of goblet and cylindrical cells expressed MUC5AC (Fig. 5C), and rare goblet cells were positive also for MUC6. MUC2 was positive in the goblet cells and in very rare cylindrical cells (Fig. 5D). Both goblet and cylindrical cells of the incomplete IM showed strong nuclear expression of CDX2, diffusely increased cell proliferation, and CD10 negativity. In one case of a 75-year-old male, the incomplete IM contained focal low-grade dysplasia. The cardiac-type mucosa around the IM showed mild chronic inflammation.
Fig. 5. Esophageal incomplete intestinal metaplasia. A: HE stained section shows intestinal metaplastic epithelium, B: alcian blue/PAS stain demonstrates mucin positivity in both cell types, C: MUC5AC positivity, D: MUC2 expression in the goblet cells and in rare cylindrical cells (A: hematoxylin and eosin, magnification x200; B: alcian blue/PAS, magnification x200; C and D: ABC technique, magnifications x100 and x400, respectively). 
Fig. 6. Expression of MUC5AC in normal intestinal mucosa. A: duodenum, B: terminal ileum, C: colon (ABC technique, magnifications x100). 
The control groups contained 50 duodenal biopsies, 50 biopsies from the terminal ileum, and 50 biopsies from various parts of the colon, respectively (Table 2). Every case included 1-3 specimens of maximum diameter 3 mm. The MUC5AC+ goblet cells were found in 77 % of the cases of normal duodenal mucosa (Fig. 6A), in 0.9 % of cases of the normal terminal ileum (Fig. 6B), and in 70 % of cases with normal colonic mucosa (Fig. 6C) (for details see Table 2). These MUC5AC+ cells were usually seen in the superficial portion of the crypt or in the superficial epithelium. Non-goblet cylindrical cells were always MUC5AC negative. MUC6 was negative in all intestinal biopsies without inflammation. The biopsies with finding of inflammation included cases of celiac disease (duodenal specimens), Crohn’s disease (terminal ileum specimens), and ulcerative colitis (colonic specimens). We have found in all of these cases MUC5AC expression in the goblet cells and, rarely, also in the cylindrical cells (mostly ++ or +++) (Fig. 7A). The positivity was seen also in the basal portion of the crypts. MUC6 was positive in rare goblet and/or cylindrical cells in the duodenum by celiac disease (Fig. 7B) and in the terminal ileum by Crohn’s disease, and it was negative in the colonic mucosa by ulcerative colitis.
2. MUC5AC- and MUC6-positive goblet cells in the small and large intestine. 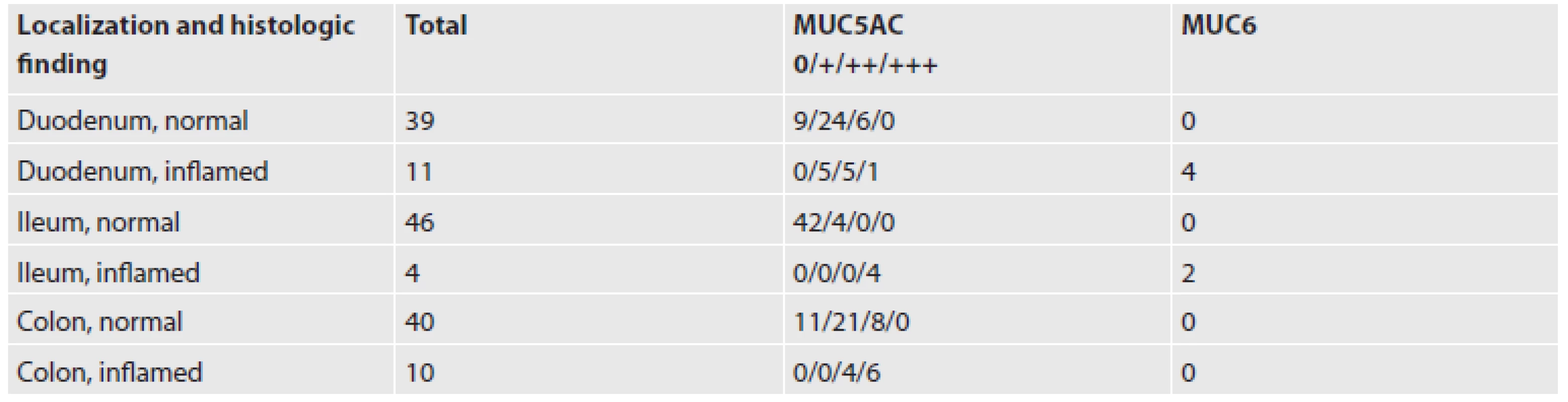
0: negative; +: from 1 to 10 MUC5AC positive cells; ++: from 10 to 20 MUC5AC positive cells; +++: more than 20 MUC5AC positive cells Fig. 7. Inflamed mucosa. A: MUC5AC in colitis ulcerosa, B: MUC6 positive cells in the duodenal mucosa by celiac disease (ABC technique, magnifications x100). 
DISCUSSION
The new classification of IM using immunohistochemical mucin markers includes two types of IM: 1. incomplete IM, i.e. mixed gastric and intestinal type, and 2. complete IM, i.e. purely intestinal type. In the stomach, IM usually originates as small microscopic foci in the antral glands, as a consequence of abnormal differentiation of the stem cells in the proliferative mucus-neck region (2,5,11). Using the immunohistochemical method, we can confirm that IM begins (in its incomplete form) in the mucus-neck region and in the nearby foveolar epithelium, and that it matures gradually in the direction of the mucosal surface. In the course of this maturation, the incomplete type changes into the complete one. Incomplete IM in the mucus-neck zone was observed already by Inada et al. (11). However, these authors did not mention further development of this IM. Immunohistochemically, incomplete IM in the mucus-neck region expresses (besides intestinal MUC2) gastric mucins, mainly MUC5AC in both goblet and cylindrical cells. In addition, in some cases it shows focal non-continuous brush border (a subtle difference from the “classical” description of incomplete IM) and increased cell proliferation (2,12). The differentiation toward the complete type is seen in the foveolae as a gradual reduction of the MUC5AC in goblet and cylindrical cells, and as the expression of MUC6 in very rare goblet cells (3,4,6). In two of our cases, the incomplete IM produced in the foveolas closely packed glands lined by epithelium with mild irregular, basally located nuclei (2,12). Metaplastic foveolar epithelium showed increased proliferation, mild p53 positivity, and negativity for AMACR. This finding corresponds with so-called atypical hyperproliferative IM (12) which was also labeled as “indefinite for dysplasia” (1,12). Our findings support the view that incomplete IM represents an initial step of the metaplastic change (6,8). As mentioned above, it starts in the mucus-neck region and it matures gradually toward the surface, transforming from an incomplete to complete type. Only in a minority of the cases does the incomplete IM not mature. In such cases, it can be seen in the surface epithelium where it represents a possible precursor of adenomatous dysplasia and intestinal-type carcinoma (3,4,6,8). In our series of 30 cases of the gastric IM, we have seen such incomplete IM on the mucosal surface in two cases only. In one of them, this IM also contained high-grade adenomatous dysplasia, and this finding supports the above-mentioned precancerous potential of such IM. Surprisingly, in our series we have found frequent positivity of gastric mucin MUC5AC in the goblet cells of the complete IM (in 33% of our cases), which contained fully differentiated and mature absorptive cells. This finding raised suspicions as to whether MUC5AC positivity is not a feature of a normal or somehow irritated epithelium of the intestine. Therefore, we examined a series of intestinal biopsies for MUC5AC. In contrast with previous reports of constant MUC5AC negativity in the intestinal mucosa by Byrd (18) and Taliano (19), we have seen MUC5AC+ goblet cells in the normal mucosa of the duodenum, terminal ileum and colon, respectively. These cells were found mostly in the superficial epithelium and in superficial portions of the crypts, and, rarely, in the bases of the crypts. Interestingly, these localizations are outside the regeneration zone of the crypt. More numerous goblet cells and rare cylindrical cells positive for MUC5AC were found in the inflamed intestinal mucosa. Such cases included the duodenum by celiac disease, terminal ileum by Crohn’s disease, and colon by ulcerative colitis. In these instances, the positive cells usually were seen in the whole length of the crypt. In cases of celiac disease and Crohn’s disease, we even found an expression of gastric mucin MUC6 in rare goblet and cylindrical cells. Of interest is that MUC6 was not found in any cases of ulcerative colitis. Our findings of MUC5AC and MUC6 in the intestinal mucosa indicate that the expression of these mucins should not be regarded a marker of immaturity. We think that for an evaluation of completeness of IM, a full differentiation of the cylindrical cells into absorptive phenotype represents a single criterion, i.e., the type of the mucin in the goblet cells is not sufficient for the diagnosis of complete or incomplete IM.
IM of the esophagus (Barrett esophagus) is currently regarded to be a premalignant condition. It develops in the metaplastic mucosa of the cardiac type (13-16,20). We, like many others, consider gastric cardia as a metaplasia of the esophageal mucosa, i.e., not as a part of the stomach. However, we included into our series of reflux esophagitis and Barrett esophagus only those cases which contained foci of the squamous epithelium on the mucosal surface. This finding proves further and more convincingly the esophageal origin of the sample (13,16). Intestinal metaplasia begins first at the most proximal region of the columnar lined esophagus adjacent to the squamocolumnar junction, and it extends distally, usually without skipping areas (13). This IM is, in contrast with IM of the stomach, almost always of the incomplete type. It contains less of a differentiated population of goblet, cylindrical and neuroendocrine cells, respectively. Rare Paneth cells may be seen, as well. Well differentiated absorptive cells are not present (21). The goblet and cylindrical cells produce gastric type mucin MUC5AC, and rare goblet cells also are positive for MUC6 (4,14,21). Intestinal mucin is constantly positive (along with gastric mucins) in the goblet cells, and it is not infrequently seen in the cylindrical cells as well. Like Warson et al. (10), we found MUC2+ cylindrical cells quite rarely. On the contrary, others found the number of these cells to be high (6,14,20). McIntire et al. (20) even consider even these cells to be a precursor of the goblet cells, and Glickman et al. think that they can represent initial IM (4).
CDX2 was in our cases strongly positive in both types of IM, as well as in low - and high-grade adenomatous dysplasia. Our results differ from those of Khor et al., who found CDX2 expression in incomplete IM to be lower than in the complete IM (6). We think that this difference can be explained by different sensitivity of antibodies and/or immunohistochemical techniques.
Cell proliferation of the esophageal incomplete IM was enhanced diffusely. For its evaluation, as well as for the diagnosis of dysplasia, it is important to be aware that the regeneration zone in the intestinalized glands is shifted from the mucus-neck region toward the base of the crypt (like in the crypts of the normal intestine) (4,15,17,22,23). This suggests that in the evolution of the dysplasia in the Barrett esophagus, the molecular abnormalities begin in basal crypt cells, and they subsequently expand to involve the superficial portion of the crypts and surface epithelium (23). Incomplete IM of the Barrett esophagus represents an unstable epithelium that is, under the influence of sustained reflux inflammation and injury, at risk for cumulative molecular alterations (15,22,23).
In conclusion, our results indicate that incomplete IM is an initial step of the metaplastic process, and this is in accordance with some previous studies (6,8). Incomplete IM can mature into complete IM, or alternatively, it can develop dysplasia or adenocarcinoma (after accumulation of molecular alterations). In addition, we found in contrast with previously published results that gastric mucins are present also in normal intestinal mucosa, and that their expression is even enhanced in inflammatory condition such as celiac disease, Crohn’s disease and ulcerative colitis. An interesting exception is the negativity for MUC6 in our cases of ulcerative colitis. The expression of MUC5AC in the complete IM and in normal or inflamed intestinal mucosa indicates that MUC5AC cannot be regarded as a marker of immaturity. In the Barrett esophagus, our results were similar to those of previous studies, except for CDX2 of which reactivity was seen also in the incomplete type of IM.
Correspondence address:
A. Chlumska, MD, CSc.
Biopticka lab.
Mikulasske nam. 4, 32600 Pilsen
Czech Republic
e-mail: chlumska@medima.cz
phone: +420-737-220-403
Sources
1. Bosman FT. World Health Organisation.; International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4th ed., Lyon: IARC Press, 2010.
2. Erkan G., Gonul Il, Kandilci U, Dursun A. Evaluation of apoptosis along with BCL-2 and Ki-67 expression in patients with intestinal metaplasia. Pathol Res Pract 2012; 208(2): 89-93.
3. Lauwers GY. Defining the pathologic diagnosis of metaplasia, atrophy, dysplasia, and gastric adenocarcinoma. J Clin Gastroenterol 2003; 36(5 Suppl.): S37-S43.
4. Glickman JN, Blount PL, Sanchez CA, et al. Mucin core polypeptide expression in the progression of neoplasia in Barrett´s esophagus. Hum Pathol 2006; 37(10): 1304-1315.
5. Inada K., Tanaka H., Nakanishi H, et al. Identification of Paneth cells in pyloric glands associated with gastric and intestinal mixed-type intestinal metaplasia of the human stomach. Virchows Arch 2001; 439(1): 14-20.
6. Khor TS, Alfaro EE, Ooi EMM, et al. Divergent expression of MUC5AC, MUC6, MUC2, CD10, and CDX-2 in dysplasia and intramucosal adenocarcinomas with intestinal and foveolar morphology: Is this evidence of distinct gastric and intestinal pathways to carcinogenesis in Barrett esophagus? Am J Surg Pathol 2012; 36(3): 331-342.
7. Mahajan D, Bennett AE, Liu X, et al. Grading of gastric foveolar-type dysplasia in Barrett´s esophagus. Mod Pathol 2010; 23(1):1-11.
8. Park do Y, Srivastava A, Kim GH, et al. Adenomatous and foveolar gastric dysplasia: distinct patterns of mucin expression and background intestinal metaplasia. Am J Surg Pathol 2008; 32(4): 524-533.
9. Voltaggio L, Montgomery EA, Lam-Himlin D. A clinical and histopathologic focus on Barrett esophagus and Barrett-related dysplasia. Arch Pathol Lab Med 2011; 135(10): 1249-1260.
10. Warson C, Van De Bovemkamp JH, Korteland-van Male AM, et al. Barrett´s esophagus is characterized by expression of gastric-type mucins (MUC5AC, MUC6) and TFF peptides (TFF1 and TFF2), but the risk of carcinoma development may be indicated by the intestinal-type mucin, MUC2. Hum Pathol 2002; 33(6): 660-668.
11. Inada K, Nakanishi H, Fujimitsu Y, et al. Gastric and intestinal mixed and solely intestinal types of intestinal metaplasia in the human stomach. Pathol Int 1997; 47(12): 831-841.
12. Li Y, Chang X, Zhou W, et al. Gastric intestinal metaplasia with basal gland atypia: a morphological and biologic evaluation in a large Chinese cohort. Hum Pathol 2013; 44(4): 578-590.
13. Chandrasoma P, Wijetunge S, DeMeester S, et al. Columnar-lined esophagus without intestinal metaplasia has no proven risk of adenocarcinoma. Am J Surg Pathol 2012; 36(1): 1-7.
14. Glickman JN, Shahsafaei A, Odze RD. Mucin core peptide expression can help differentiate Barrett´s esophagus from intestinal metaplasia of the stomach. Am J Surg Pathol 2003; 27(10): 1357-1365.
15. Yantiss RK. Diagnostic challenges in the pathologic evaluation of Barrett esophagus. Arch Pathol Lab Med 2010; 134(11): 1589-1600.
16. Takubo K, Aida J, Naomoto Y, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum Pathol 2009; 40(1): 65-74.
17. Vieth M, Langner C, Neumann H, Takubo K. Barrett´s esophagus. Practical issues for daily routine diagnosis. Path Res Pract 2012; 208(5): 261-268.
18. Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer and Metastasis Rev 2004; 23(1-2): 77-99.
19. Taliano RJ, LeGolvan M, Resnick MB. Immunohistochemistry of colorectal carcinoma: current practice and evolving applications. Hum Pathol 2013; 44(2): 151-163.
20. McIntire MG, Soucy G, Vaughan TL, et al. MUC2 is a highly specific marker of goblet cell metaplasia in the distal esophagus and gastroesophageal junction. Am J Surg Pathol 2011; 35(7): 1007-1013.
21. Chlumská A, Mukenšnabl P, Dušek M, Mareček P, Zámečník M. Intestinální metaplazie žaludku a jícnu. Morfologické nálezy a jejich prognostický význam. Kongresové noviny, No 1, p.6. Meeting of Czech and Slovak Gastroenterologists, Nov 22, 2012, Karlovy Vary, Czech Republic.
22. Moyes LH, Going JJ. Still waiting for predictive biomarkers in Barrett´s oesophagus. J Clin Pathol 2011; 64(9): 742-750.
23. Zhang X, Huang Q, Goyal RK, Odze RD. DNA ploidy abnormalities in basal and superficial regions of the crypts in Barrett´s esophagus and associated neoplastic lesions. Am J Surg Pathol 2008; 32(9): 1327-1335.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2014 Issue 3-
All articles in this issue
- Extraintestinal oxyuriasis – report of three cases and review of literature
- Up-to-date experience with the international classification system Bethesda 2010 for thyroid fine-needle aspirate: a review
- A complex diagnostic approach in lymphomas: practical aspect in short case reports
- Molecular testing in malignant melanoma
- Soft tissue tumors - the view of the molecular biologist
- Intestinal metaplasia of the stomach and esophagus: an immunohistochemical study of 60 cases including comparison with normal and inflamed intestinal mucosa
- Myxoid variant of peritoneal epithelioid malignant mesothelioma. A case report
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Intestinal metaplasia of the stomach and esophagus: an immunohistochemical study of 60 cases including comparison with normal and inflamed intestinal mucosa
- Soft tissue tumors - the view of the molecular biologist
- Up-to-date experience with the international classification system Bethesda 2010 for thyroid fine-needle aspirate: a review
- A complex diagnostic approach in lymphomas: practical aspect in short case reports
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career















