-
Medical journals
- Career
HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
Authors: Petr Kastner; Kateřina Burdová; Pavla Pilařová
Authors‘ workplace: Faculty of Pharmacy in Hradec Králové, Charles University, Department of Pharmaceutical Chemistry and Drug Control, Hradec Králové, Czech Republic
Published in: Čes. slov. Farm., 2015; 64, 215
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Nowadays, HPLC is the most widely spread and powerful analytical tool in drug control. It is the first choice method for the solution of all problems connected with the evaluation of related substances and the assay of the active substance as well as the preservative. This separation method was thus used to develop an analytical method which enables evaluation of oral liquid drug preparations containing sodium picosulfate as the favoured laxative drug.
Experimental method
HPLC separation of components was achieved with LiChroCART®, 250 ×⋅ 4.0, Purospher® STAR, RP – 18 C, 5 μμm column using a UV detector at 263 nm. The mobile phase consisted of a buffer, acetonitrile and isopropylalcohol in the ratio of 55 : 43 : 2 (v/v/v). The buffer contained disodium hydrogen phosphate, water R and cetyltrimethylammonium bromide. The pH value was adjusted by phosphoric acid to 7.0. The temperature of the column was 40 °C, injection volume 4 μμμl, the flow rate was adjusted to 1.0 ml/min.
1. A typical chromatogram of a pharmaceutical preparation containing sodium picosulfate as the active ingredient, sodium benzoate as the preservative and impurity A, which is the main hydrolytical degradation product of the active substance 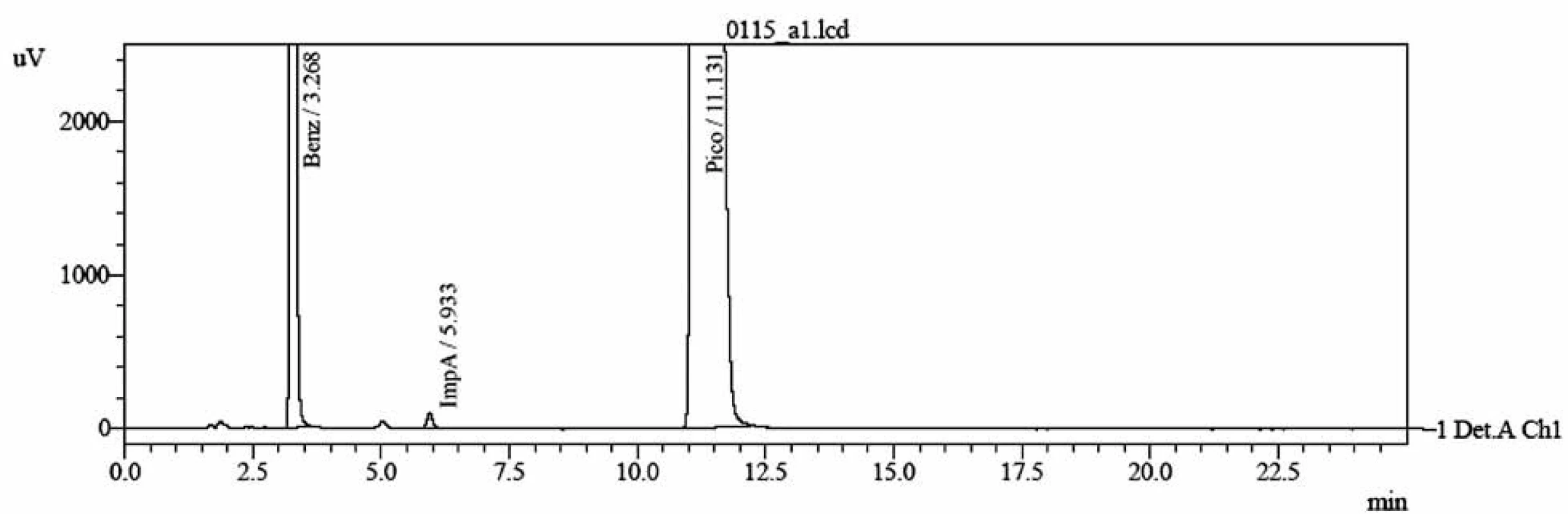
Results and discussion
The developed method was based on a valid pharmacopoeial one1), it was optimized for and then it was validated2) as sufficiently selective, precise, accurate, linear and sensitive. Robustness was tested by means of the Plackett-Burman design3) and it was found that the ratio of acetonitrile in the mobile phase exerts the greatest influence on separation from all proved parameters. On the other hand, flow, buffer concentration and the ratio of propan-2-ol in the mobile phase little affect the separation.
Conclusions
The developed method enables simultaneous evaluation of the content of the active ingredient, its related substances and the preservative. It was sufficiently validated from the standpoint of selectivity, linearity, precision, accuracy, sensitivity and robustness, and can be used for release control of the pharmaceutical preparation and for its stability studies, too.
This work was supported by a research project SVV 260062.
Conflicts of interest: none.
PharmDr. Petr Kastner, Ph.D.
Faculty of Pharmacy
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: kastner@faf.cuni.cz
Sources
1. European Pharmacopoeia. 8th ed. Strasbourg: Council Of Europe, 2013.
2. ICH [online] 2013 [cit. 2013-12-11]. Available from: http://www.ich.org/products/guidelines/quality/qualitysingle/article/ validation-of-analytical-procedures-text-and-methodology.html
3. Holík M. Optimalizace analytických postupů pomocí Plackettova-Burmanova plánu. Chemické listy. Praha: Česká společnost chemická 2004; 98.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2015 Issue 5-
All articles in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- Drugs and health care expenditure on the aging population
- Drug bioavailability increasing by formulation of liquisolid systems
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

