-
Medical journals
- Career
The possible involvement of intestine-derived IgA1: a case of IgA nephropathy associated with Crohn’s disease
Authors: Tomohiro Terasaka 1; Haruhito A. Uchida 1,2*; Ryoko Umebayashi 1; Keiko Tsukamoto 1; Keiko Tanaka 1; Masashi Kitagawa 1; Hitoshi Sugiyama 1,3; Hiroaki Tanioka 4; Jun Wada 1
Authors‘ workplace: Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, -5-1 Shikata-cho, Kita-ku, Okayama 700-8558, Japan. 1; Department of Chronic Kidney Disease and Cardiovascular Disease, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama 700-8558, Japan. 2; Department of Human Resource Development of Dialysis Therapy for Kidney Disease, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama 700-8558, Japan. 3; Department of Medicine Oncology, Okayama Rosai Hospital, 1-10-25 Chikkomidori-machi, Minami-ku, Okayama 702-8055, Japan. 4
Published in: BMC Nefrol 2016, 17:122
Category: Case report
doi: https://doi.org/10.1186/s12882-016-0344-1© 2016 The Author(s).
Open access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The electronic version of this article is the complete one and can be found online at: http://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-016-0344-1Overview
Background:
A link between IgA nephropathy and Crohn’s disease has recently been reported. Other researchers hypothesize that intestine-derived IgA complexes deposit in glomerular mesangial cells, eliciting IgA nephropathy. Intestinal mucosal plasma cells mainly secrete IgA2. Nevertheless, IgA1 deposition is strongly implicated as being the primary cause of IgA nephropathy.Case presentation:
A 46-year-old Japanese man developed IgA nephropathy 29 years ago, following tonsillectomy. As a result, a normal urinalysis was obtained. The patient previously suffered Crohn’s disease followed by urinary occult blood and proteinuria six years ago. Exacerbation of IgA nephropathy was highly suspected. Therefore a renal biopsy was performed. A diagnosis of exacerbation of IgA nephropathy with mesangial cell proliferation and fibrotic cellular crescent was based upon the pathological findings. The patient exhibited a positive clinical course and eventually achieved a remission with immunosuppressive therapy including prednisolone treatment. Immunostaining for the detection of IgA subtypes was performed on both of his kidney and excised ileum. The results revealed IgA1 and IgA2 deposition by submucosal cells in intestine. Furthermore, IgA1 deposition of mesangial areas in the patient’s kidney, indicated an association of IgA1with the exacerbation of IgA nephropathy.Conclusion:
This case represents the possibility that the intestine-derived IgA1 can be the origin of galactose-deficient IgA which is known to cause IgA nephropathy exacerbation.Keywords:
Crohn’s disease, IgA nephropathy, IgA1, IgA2, Inflammatory bowel diseaseBackground
IgA1 is one of the subtypes of IgA produced in bone marrow, tonsils and respiratory tracts. Also, it can be found predominantly in the glomeruli of patients with IgA nephropathy. This observation supports the idea of using tonsillectomy as an effective therapeutic intervention for IgA nephropathy. Alternatively, intestinal mucosal plasma cells mainly secrete IgA2 (approximately 60 % in mucosal cells), another subtype of IgA, rather than IgA1 [1].
Recent reports have indicated a strong link between IgA nephropathy and Crohn’s disease. Several hypotheses exist, indicating this, however the underlying mechanism is still unknown. One can speculate that intestine-derived IgA is possibly deposited in glomerular mesangial cells, eliciting IgA nephropathy [2, 3,4]. Here, we present a case report of a patient suffering from exacerbated IgA nephropathy associated with Crohn’s disease, who had tonsillectomy 29 years ago. We performed immunohistochemical analysis in renal biopsy and excised ileum specimen to determine whether it was IgA1 or IgA2 that was specifically associated with the exacerbation of IgA nephropathy. The results indicated that IgA1, not IgA2, is the causative source of IgA nephropathy exacerbation. This indicates a link between inflammatory intestine-derived IgA1 and IgA nephropathy.
Case presentation
A 46-year-old Japanese man suffered from IgA nephropathy, prior to be given tonsillectomy 29 years ago. Urinalysis revealed neither hematuria nor proteinuria after surgical removal of his tonsils. The patient experienced a sudden onset of Crohn’s disease 6 years ago. As a result, he was administered several medications to alleviate the symptoms of Crohn’s disease; initially, 5-aminosalicylic acid (ASA). Due to the unstable state of Crohn’s disease, an ileum resection was performed. Additionally, prednisolone, azathioprine (AZA) and infliximab were eventually incorporated in his treatment. Despite therapeutic drug intervention, his abdominal symptoms including diarrhea persisted. Consequently, he was then referred to our hospital because of proteinuria and urinary occult blood. An otolaryngologic check-up revealed no sinusitis, and residual tonsil and gross physical examination provided no remarkable findings. Abdominal CT displayed bilateral slightly atrophic kidneys. A laboratory workup showed a mild renal dysfunction (creatinine; 1.37 mg/dl, creatinine clearance; 69.1 ml/min), without any other abnormal findings of blood (IgG, 1492.3 mg/dl; IgA, 223.2 mg/dl; IgM, 33.8 mg/dl; C3, 101.4 mg/dl; C4, 22.7 mg/dl; CRP, 0.09 mg/dl, anti-streptolysin O, 109 IU/ml; normal range: 0–239; anti-streptokinase, ×1280; normal: < ×2560). Serologic tests for hepatitis B and C and HIV were all negative. Urinalysis showed proteinuria (2+) and occult blood (2+), with 5–9 red blood cells and < 1 white blood cells per high-power field in sediment. Twenty four-hour urine collection revealed a protein level of 1.44 g/day. To detect occult blood (1+ to 2+) and continuous proteinuria (2+ to 3+), a renal biopsy was performed to examine whether IgA nephropathy relapsed or other glomerular diseases occurred. Immunofluorescent examination of mesangial cells exhibited intense (3+) mesangial granular staining of IgA (Fig. 1a). Moderate (2+) granular staining of IgM and C3 in the mesangial area was observed. Examination by light microscopy revealed expansion of mesangial cells and fibrotic cellular crescents (Fig. 1b). These findings are consistent with IgA nephropathy. Given these observations, prednisolone of 40 mg/day was started, with 10–20 % reduction monthly and with every subsequent visit. After 1-year of treatment (5 mg/day dosage of prednisolone is continued), his urine protein levels decreased to 0.1 to 0.3 g/gCr, with no occult blood was detected.
1. Histological findings of the renal tissues. a IgA, (b) Mesangial matrix expansion and fibrocellular crescent formations were observed in glomeruli with periodic acid-Schiff (PAS) staining (400×). c Slight changes of interstitial fibrosis and arteriole sclerosis with Masson staining (40×) 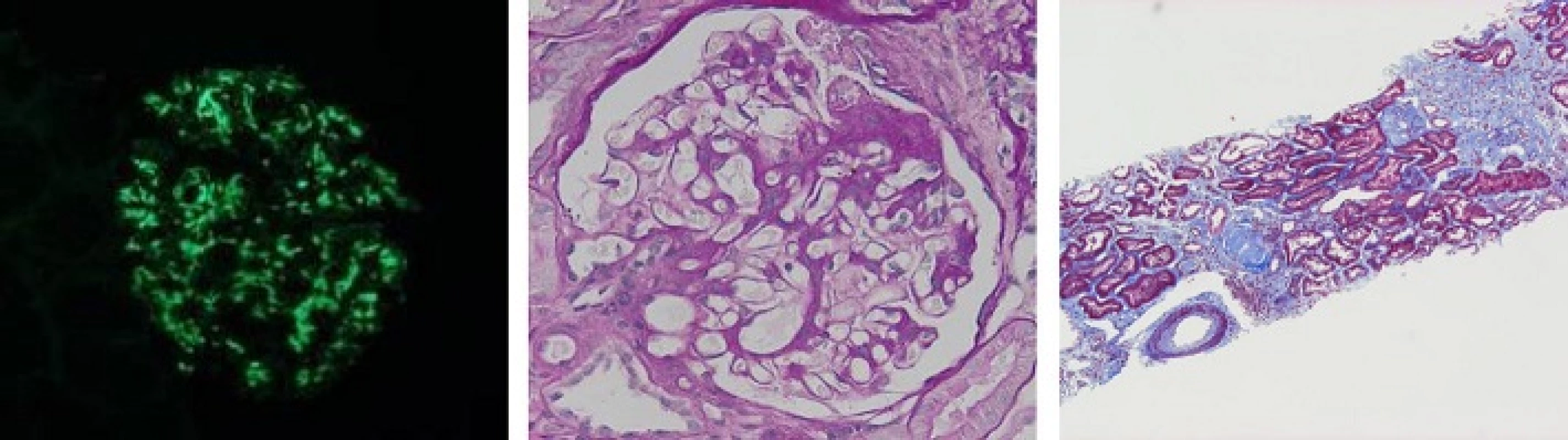
Due to his clinical course, we strongly suspected the association between the occurrence of intestinal inflammatory disease and the exacerbation of IgA nephropathy. Based on the predominance of IgA2subtype found in the intestinal immune system, the patient’s excised ileum and biopsied kidney species were examined to determine whether IgA2 subtype might be mainly involved in the exacerbation of IgA nephropathy. Immunohistochemical staining demonstrated that lymphocytes and plasma cells had infiltrated on the inflammatory ileum mucosa. In particular, at the layer of submucosa, total IgA, IgA1 and IgA2 positive cells were widely distributed, a number of IgA1 positive cells were observed as well as IgA2positive cells (Fig. 2). Alternatively, the patient’s kidney sample demonstrated that IgA1 was mainly deposited granular to mesangial areas (Fig. 3). These results raised the possibility that inflammatory ileum derived IgA1in our patient suffering from Crohn’s disease was in fact associated with the exacerbation of IgA nephropathy.
2. Immunohistological findings of the intestine tissues. Exacerbated intestine mucosal infiltration of lymphocytes and plasma cells with Hematoxylin-Eosin staining (a). Immunostaining of total IgA (b), IgA<sub>1</sub> (c), IgA<sub>2</sub> (d) in submucosal layer of intestine 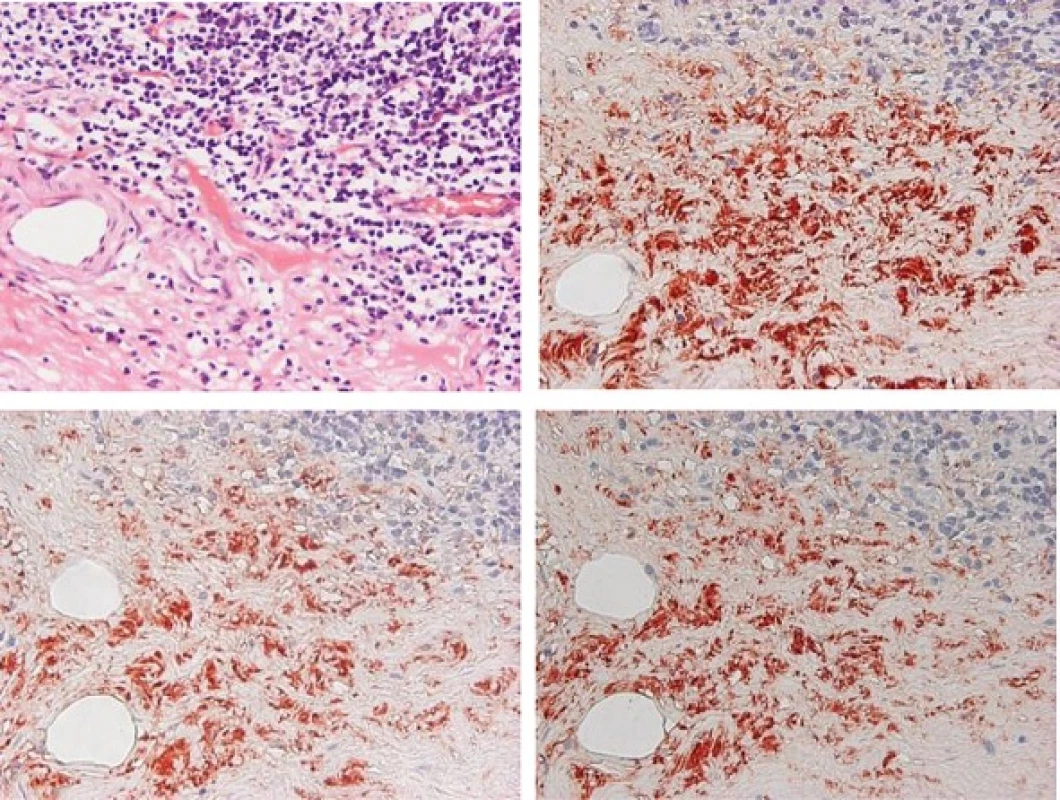
3. Immunofluorescent histological findings of the glomeruli. Immunostaining of total IgA (a), IgA<sub>1</sub> (b), IgA<sub>2</sub> (c) in kidney glomeruli 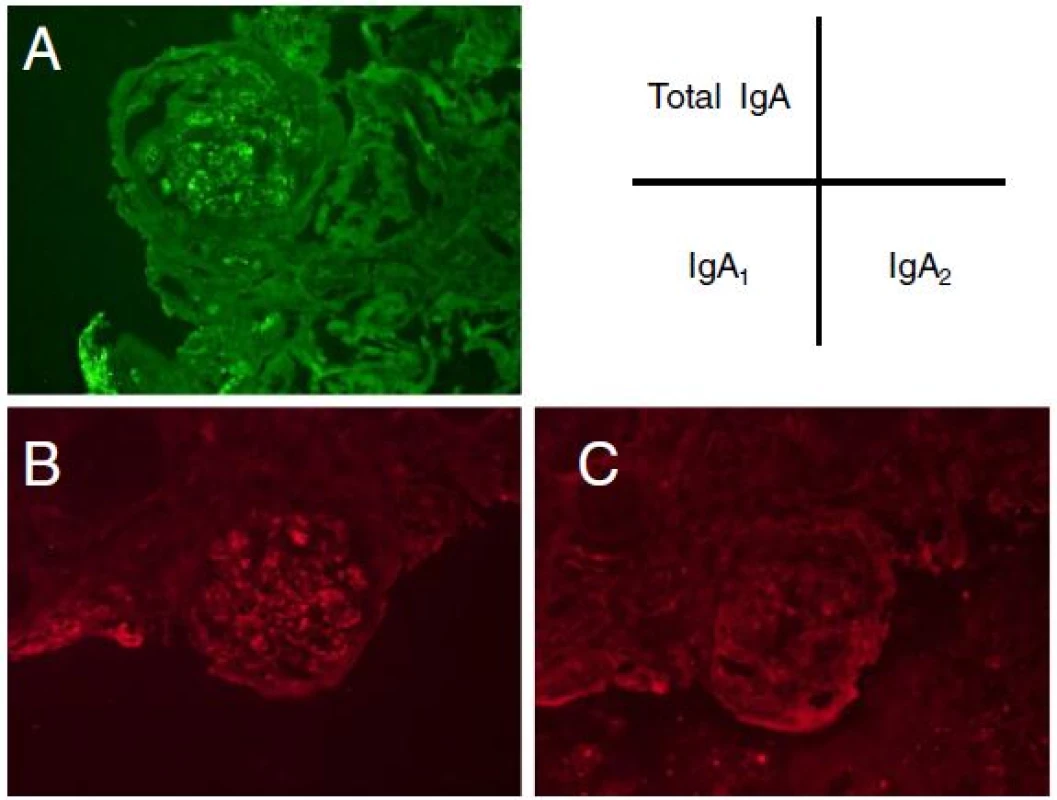
Conclusions
Renal or urologic complications are common in Crohn’s disease (up to 25 % of diagnosed patients) [5]. However, most of patients affected with Crohn’s disease exhibit urologic complications, such as nephrolithiasis with calcium oxalate or urate stones, enterovesical fistulas and ureteral obstruction [6]. Renal parenchymal dysfunction of tubulointestitial nephritis (TIN) occurs in 1 out of 500 patients who undertake 5-ASA, which is one of the key drugs prescribed for inflammatory bowel disease (IBD) [7]. 5-ASA induced TIN is reported to appear mostly within 12 months of the drug administration, but the prolonged phase of 5-ASA treatment also can cause TIN. Early and definite diagnosis of nephropathy with IBD should be confusing because their cases are generally medicated with 5-ASA for a long time.
IgA nephropathy is the most common type of glomerulonephritis and it is caused by primary or secondary pathogen disease such as seronegative arthritis, cirrhosis, celiac disease, vasculitis and HIV [8]. Although the effect of tonsillectomy on IgA nephropathy is still controversial, several reports suggest its practical efficacy at the present time. The retrospective study showed that tonsillectomy had an effect on renal outcome [9]. The decrease level of proteinuria in steroid pulse therapy with or without tonsillectomy was significant [10]. Reports of IgA nephropathy associated with Crohn’s disease are increasing. A retrospective review of 83 kidney biopsies with IBD report that the most common diagnosis was IgA nephropathy (24 %), followed by interstitial nephritis (19 %) which was suspected of 5-ASA relation, and arterionephrosclerosis (12 %) [11]. In that review, it was not clearly mentioned whether the occurrence of IgA nephropathy in Crohn’s disease was different from that in ulcerative colitis. The mechanism of the complication between IgA nephropathy and Crohn’s disease has been discussed in three points. First, the systemic absorption of IgA at the intestine mucosal inflammation site is associated with IgA nephropathy [2, 3, 4]. Second, the possible involvement of a common genetic factor; especially human leukocyte antigen (HLA)-DR1 has been the suspected factor that provides a link between Crohn’s disease and IgA nephropathy [12, 13]. Third, abnormal T helper lymphocyte is a factor that may contribute to the pathogenesis associated with these two diseases [14].
Further experiments are needed to confirm these hypotheses. On the other hand, in Crohn’s disease, intestinal mucosal inflammation is thought to lead to the systemic absorption of antigens and bacteria, and high serum levels of IgA and IgG [2]. Alternatively, increased intestinal mucosal permeability was demonstrated in IgA nephropathy [15]. The association of the etiology of Crohn’s disease after tonsillectomy has been reported [16], yet, it is not clear whether this association is applicable to our case or not. During the term of exacerbation of bowel disease, IgA nephropathy deteriorates or clinically evoked. Treatment of bowel disease with either immunosuppression therapy or bowel resection is associated with a clinical remission of IgA nephropathy [17]. These clinical observations support that a high level of systemic IgA derived from the ileum tract leads to the onset of IgA nephropathy. We hypothesized that intestine-derived IgA2 would react to this mechanism, since mucosa of lower gastrointestinal tract secreted mostly IgA2 than IgA1. Nonetheless, in this case, IgA1 deposition, but not IgA2 deposition, in kidney glomerular mesangial sites was observed. The galactose-deficient IgA1 is recognized as being the likely cause of IgA nephropathy [18, 19]. IgA1 producing cells are known to increase in the intestine of IBD [20], which also provides the potential answer to why intestine-derived abnormal sugar-chain IgA1 is a causative agent of IgA nephropathy. In conclusion, there appears to be a link between Crohn’s disease, IgA nephropathy and galactose-deficient IgA1 secreted by the intestine, and all may be confounding factors that contribute to the onset or exacerbation of IgA nephropathy.
Acknowledgements
We would thank Ms. Nahoko Iwata for her technical assistant for renal biopsy and Dr. Terri Stoner for editing the manuscript of this case report.
Funding
This study was not funded by any third party.
Availability of data and materials
All data and material were presented in this manuscript.
Authors’ contributions
Designated authors meet all four criteria for authorship in the ICMJE recommendations. Individual contribution of each authors are as follows. TT, HAU, HT and KT made decisions on patient’s examinations and therapies. TT, HAU and RU reviewed previous publications and wrote the whole manuscript. KT, MK are nephrologists at Okayama University Hospital and performed the renal biopsy of the patient. TT and RU performed the immunohistologic examinations of IgA nephritis of the patient. HAU is an associate professor of Chronic Kidney Disease and Cardiovascular Medicine at Okayama University Graduate School and supervised the manuscript. HT is a gastroenterologist at Okayama Rosai Hospital and treated Crohn’s disease of the patient. TT, HAU, RU, KT, KT, MK, HS, HT and JW contributed to the interpretation of the etiology and discussion. HS is a professor of Human Resource Development of Dialysis Therapy for Kidney Disease at Okayama University Graduate School. JW is a professor of Nephrology, Rheumatology, Endocrinology and Metabolism, at Okayama University Graduate School and supervised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication.
Ethics approval and consent to participate
This study procedure was performed in accordance with the ethical standards of the Committee on Publication Ethics (COPE), the World Association of Medical Editors (WAME) Policy Statement on Geopolitical Intrusion on Editorial Decisions and the Declaration of Helsinki.
Abbreviations
ASA, 5-aminosalicylic acid; AZA, Azathioprine; HLA, Human leukocyte antigen; IBD, Inflammatory bowel disease; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; TIN, Tubulointerstitial nephritis
Received: 17 March 2016
Accepted: 1 September 2016
Published: 5 September 2016* Correspondence:
Haruhito A. Uchida
1Department of Nephrology, Rheumatology, Endocrinology and Metabolism,
Okayama University Graduate School of Medicine,
Dentistry and Pharmaceutical Sciences,
2-5-1 Shikata-cho, Kita-ku,
Okayama 700-8558, Japan2Department of Chronic Kidney Disease and Cardiovascular Disease,
Okayama University Graduate School of Medicine,
Dentistry and Pharmaceutical Sciences,
2-5-1 Shikata-cho, Kita-ku,
Okayama 700-8558, Japanhauchida@okayama-u.ac.jp
Sources
1. Chiba M, et al. IgA1 & IgA2 distribution in the intestine. Gastroenterol Jpn. 1987;22 : 18–23.
2. Mahoney S, et al. Systemic and mucosal antibodies to Klesiella in patients with ankylosing spondylitis and Crohn’s disease. Ann Rheum Dis. 1992;51 : 1296–300.
3. Pouria S, et al. Secondary IgA nephropathy. Semin Nephrol. 2008;28 : 27–37.
4. Pipili C, et al. Is there aney association between IgA nephropathy, Crohn’s disease and Helicobacter pylori infection? Ren Fail. 2012;34 : 506–9.
5. Rothfuss KS, et al. Extraintestinal manifestations and complications of inflammatory bowel diseases. World J Gastroenterol. 2006;12 : 4819–31.
6. Pardi DS, et al. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93 : 504–14.
7. Waters AM, et al. Tubulointerstitial nephritis as an extraintestinal manifestation of Crohn’s disease. Nat Clin Pract Nephrol. 2008;4 : 693–7.
8. Forshaw MJ, et al. IgA nephropathy in association with Crohn’s disease. Int J Color Dis. 2005;20 : 463–5.
9. Xie Y, et al. The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 2003;63 : 1861–7.
10. Kawamura T, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29 : 1546–53.
11. Ambruzs JM, et al. The Histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9 : 265–70.
12. Friedman BI, et al. HLA associations in IgA nephropathy and focal and segmental glomerulosclerosis. Am J Kidney Dis. 1994;23 : 352–7.
13. Toyoda H, et al. Distinct associations of HLA class II genes with inflammatory bowel disease. Gastroenterology. 1993;104 : 741–8.
14. Choi JY, et al. A case of rapidly progressive IgA nephropathy in a patient with exacerbation of Crohn’s disease. BMC Nephrol. 2012;13 : 84.
15. Davlin JC, et al. Increased intestinal permeability to (51Cr) EDTA is correlated with IgA immune complex-plasma levels in children with IgA-associated nephropathies. Acta Paediatr Scand. 1988;77 : 118–24.
16. Maté-Jimenez J, et al. Tonsillectomy and inflammatory bowel disease location. Eur J Gastroenterol Hepatol. 1996;8 : 1185–8.
17. Filiopoulos V, et al. IgA nephropathy in association with Crohn’s disease: a case report and brief review of the literature. Ren Fail. 2010;32 : 523–7.
18. Hiki Y, et al. Underglycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy. J Am Soc Nephrol. 1999;10 : 760–9.
19. Tomana M, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104 : 73–81.
20. Kett K, et al. Local IgA subclass alterations in ulcerative colitis and Crohn’s disease of the colon. Gut. 1987;28 : 1013–21.
Labels
Paediatric nephrology Nephrology
Article was published inBMC Nephrology

2016 Issue 122
Most read in this issue
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

