-
Medical journals
- Career
SUTURE VERSUS FIBRIN GLUE MICRONEURAL ANASTOMOSIS OF THE FEMORAL NERVE IN SPRAGUE DEWLY RAT MODEL. A COMPARATIVE EXPERIMENTAL ASSESSMENT OF THE CLINICAL, HISTOLOGICAL AND STATISTICAL FEATURES
Authors: M. Adel 1; D. Abdo Elgamal 2; R. Bakry 3; M. Abdelkader 4; M. Elshazly 1; A. Kamel 1
Authors‘ workplace: Department of Plastic Surgery, Assiut University Hospital, Egypt 1; Department of Histology, Faculty of Medicine, Assiut University, Egypt 2; Department of Clinical Pathology, South Egypt Cancer Institute, Assiut University, Egypt 3; Department of General Surgery, Assiut University Hospital, Egypt 4
Published in: ACTA CHIRURGIAE PLASTICAE, 59, 2, 2017, pp. 65-71
INTRODUCTION
Severe nerve injury has a devastating impact on patients’ quality of life. Typical symptoms are sensory and motor function defects that can result in complete paralysis of the affected limb or development of intractable neuropathic pain. Nerve fibres of the transected nerve regenerate spontaneously to the extent limited by the size of the nerve gap, neuroma, and scar tissue formation2. Although considered a standard method in repairing peripheral nerve lesions, nylon thread suture may cause an inflammatory reaction that may affect the regeneration process and be difficult to perform in small-calibre nerves3. To improve the functional outcomes of peripheral nerve repair, a suture free seam with synthetic adhesive has been proposed as an alternative to micro-sutures for achieving proper coaptation of the nerve endings. Using fibrin glue in nerve repair in a rat model had provided better conditions for regeneration than suture after sciatic nerve transection in previous studies4. In our study, we are trying to extensively analyse the alternatives in many new aspects and after a longer postoperative period to have a wider scale of evaluation.
MATERIAL AND METHODS
This study was approved by the local ethical committee of Faculty of Medicine at Assiut University. A total of 70 Sprague Dawley rats were used for this study, weighing between 250 g and 300 g. The rats were divided into three groups. Group one consisted of 30 rats, in which the sciatic nerve was transected and immediately repaired with 10-0 suture (Daclon-SMI AG-Belgium). Group two consisted of 30 rats in which the sciatic nerves were transected and repaired by fibrin glue. Control group included 10 rats.
The rats were operated under general anaesthesia with intra-peritoneal Sodium Thiopental 500 mg (Farcopental-Pharco Pharmaceuticals-Egypt) in a dose of 0.03 mg 0.04 mg/g of body weight5.
The dorsal aspect of both hind limbs to the midback was prepared by shaving and Betadine (Betadine–Nile Company–Egypt) washing. A dorso-lateral incision was made starting 0.5 cm laterally from the animal’s midline and extending laterally for three cm towards the tibiofemoral articulation.
The femoral biceps and gluteus muscle were separated using blunt dissection to allow exposure of the sciatic nerve; transection was performed as shown in Figure 1.
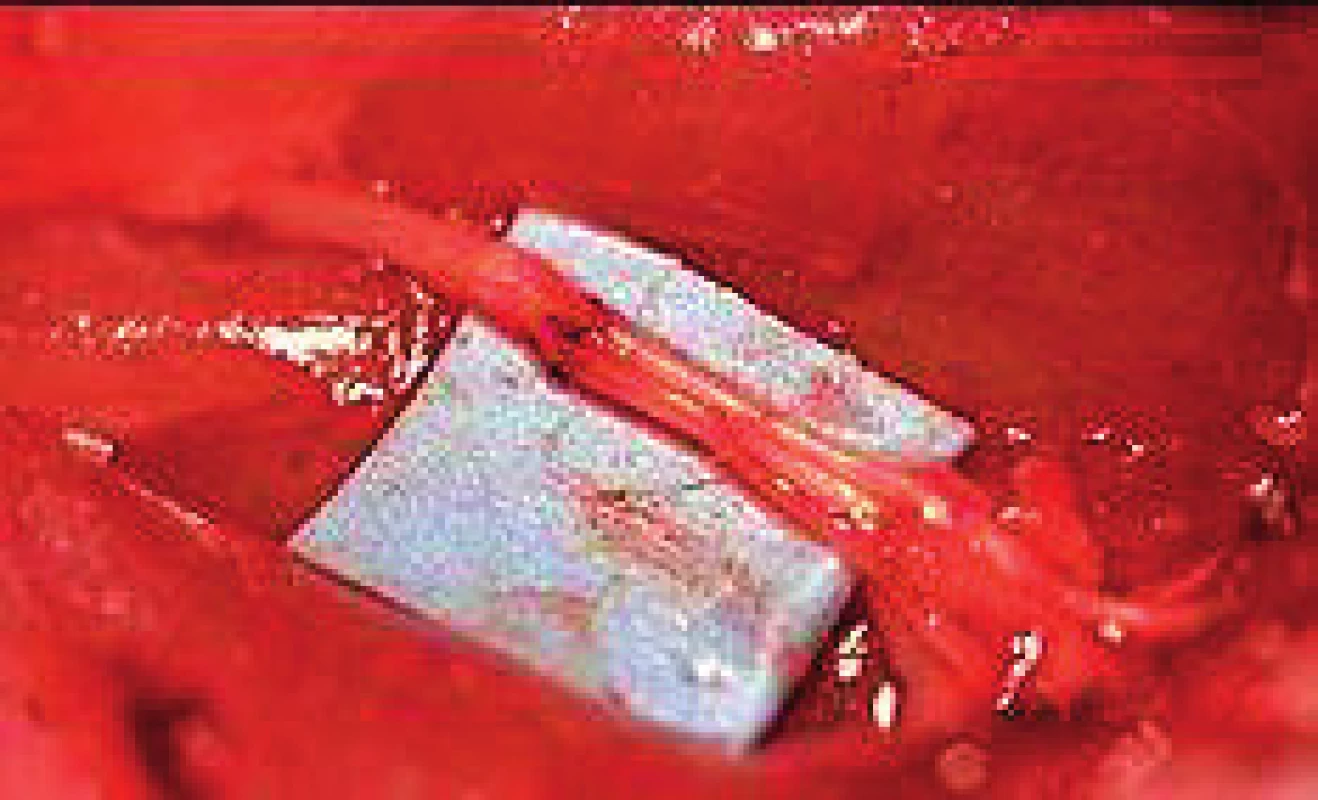
1. Exposed transected sciatic nerve 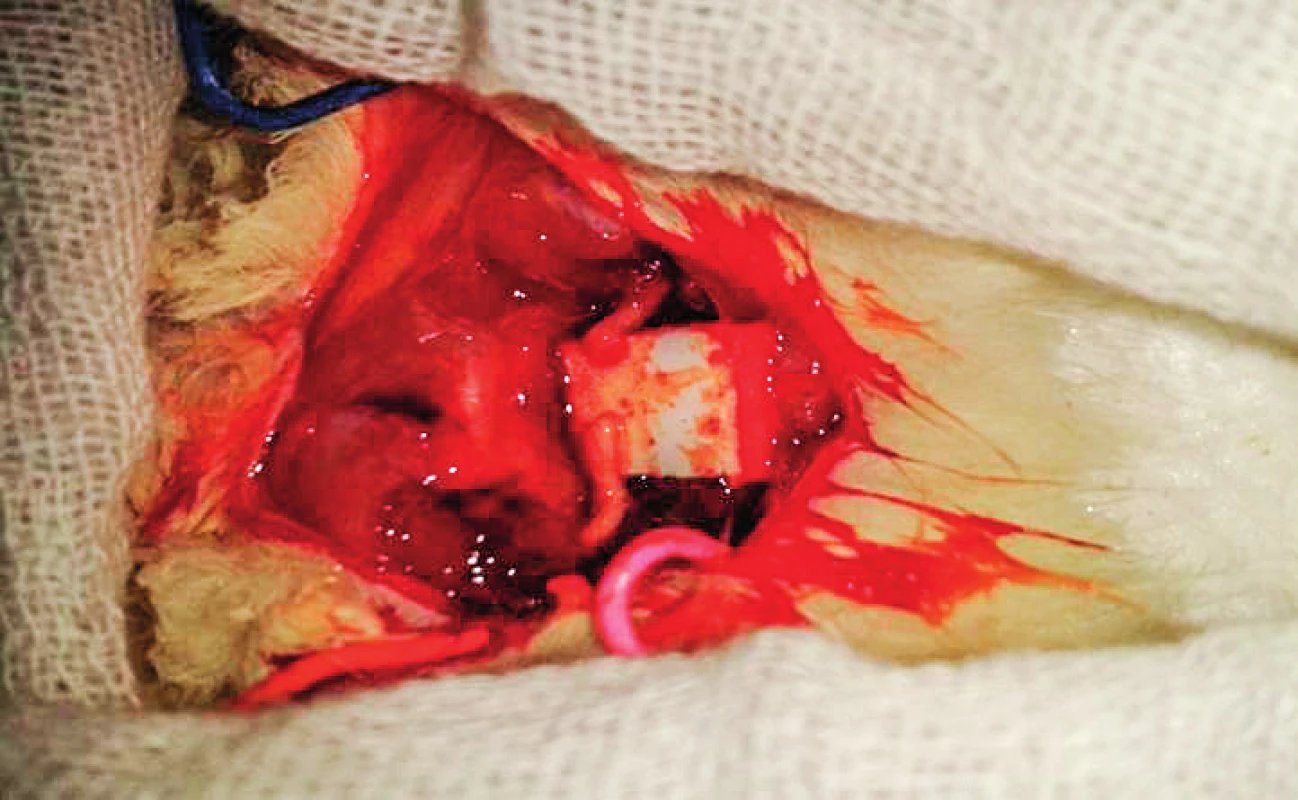
In group one, cut ends of the nerve were repaired using the operating microscope (LEICA MS 5) with interrupted epineural micro-sutures using 10-0 monofilament polyamide spatulated suture as shown in Figure 2 with 220 μm diameter, 6.4 mm length, 3/8 circle needle (Daclon-SMI AG-Belgium).
In group two, the transected nerves were placed on a small piece of latex glove material and glued with fibrin glue, which was prepared at The Clinical Pathology Department of South Egypt Cancer Institute. It is mainly composed of Fibrinogen concentrate (vial 1); Thrombin containing 4.9 mg of dry thrombin was dissolved in 1 ml of calcium chloride solution (vial 2) as shown in Figure 3. It was applied as two drops of fibrinogen sealant solution followed by the thrombin solution over the aligned nerve stumps held with two Jweler forceps. Care was taken that a maximum contact area between nerve ends was obtained and the stumps were accurately coapted. Stabilization of the nerve ends was maintained for two minutes, the latex material was removed after five minutes. In the control group (10 cases) a piece of 15 mm of the sciatic nerve was carefully dissected and harvested without any further manipulations to be studied as a histological standard landmark in comparison to the other groups.
Fig. 3A. Holding the transected nerve ends from the epineurium and surrounding tissue 
Fig. 3B. Fibrin glue application 
Fig. 3C. Complete healing with fibrin glue 
Animals were followed for up to 30 days. After the follow up period, gross appearance of nerves was observed for any dehiscence or neuroma formation. A 15 mm segment was excised including the anastomotic site and it was preserved in 10% formaldehyde solution and the specimens were processed for the following:
Histological evaluation
The specimens were fixed in 10% buffered formalin solution for 48 hours, and they were then dehydrated in ascending grades of ethanol and embedded in paraffin. Serial sections of 6–7 thickness were cut and subjected to Haematoxilin and Eosin stain for histological examination with light microscope. Masson Trichrome stain was also used to demonstrate extent of fibrosis.
Morphometric analysis
The studied groups were subjected to morphometric analysis using an image-analysing system software (Leica Q 500 MCO, Germany) at the Histology Department, Faculty of Medicine, Assiut University. Epineural thickness, cross-sectional diameter of the nerve and scar tissue formation index (epineural thickness dividing the whole cross sectional diameter) were measured6. The previously mentioned parameters were determined in H&E-stained sections at 40x magnification in ten non-overlapping fields of the entire section.
Statistical analysis
The anastomotic time (the time required to perform the anastomosis) was recorded in both techniques. Data were expressed as mean ±SD. Comparison between groups was done using Mann-Whitney test, using SPSS program version 19 (SPSS Inc., Chicago, Illinois, USA). P value less than 0.05 was considered significant.
RESULTS
In the first and second group, there was ulceration at the affected limb and adhesions with the surrounding muscles, which were nearly equal in both groups and were dissected carefully. There were no signs of infection or inflammation. There was one case of neuroma formation (localized swelling at the anastomotic site) in group one. In group two there was one case of partial wound dehiscence.
Histological evaluations
Nerve structure: In group one, nerve trunks were preserved but with marked axonal degeneration in the nerve fibres at and distal to the anastomotic site; there is also a marked increase in the number of leukocytes and other inflammatory cell infiltrate as shown in Figure 4A. In group two, examination revealed that the nerve trunks were preserved as a whole with smooth continuous more organized nerve fibres as compared with group one, but with smaller shrunken fascicle as shown in Figure 4B.
Fig. 4A. In group one the nerve structure as a hole was preserved but with axonal degeneration (arrow head) and inflammatory cell infiltration (*) 
Fig. 4B. In group two there was increase of perineural blood vessels (arrow) and irregular shrunken blood vessels (F) 
Degenerative changes: In group one, there was intensive endoneural hypercellularity with Schwann cell proliferation and multinucleated giant cell formation as shown in Figure 5A. In group two, there was better nerve fibre organization and continuity with minimal Schwann cell proliferation as compared with group one as shown in Figure 5B. In group one, there was also marked endoneural oedema and marked axonal degeneration as shown in Figure 5C. In group two, a magnified transverse section of sciatic nerve in group two showing decreased oedema and axonal degeneration as compared with group one though inflammatory cell infiltration may still exist as shown in Figure 5D.
Fig. 5A. In group one with higher magnification there was intense endoneural hypercellularity (*), Schwann cell proliferation (↑) H&E X 400, and Multinucleated giant cell formation (arrow head). Inset X1000 
Fig. 5B. In group two a longitudinal section of sciatic nerve showed better nerve fibre organization and minimal Schwann cell proliferation compared with group one. H&E X400 
Fig. 5C. In group one there was also intense endoneural oedema H&E X 400 
Fig. 5D. In group two a magnified transverse section of sciatic nerve showed decreased oedema and axonal degeneration compared with group one though inflammatory cell infiltration may still exist (*) H&E X400 
Blood vessels changes: In group one, there were apparently small calibre blood vessels compared to the control group as shown in Figure 6A, while in group two there were significantly remarkably dilated congested blood vessels with apparent increase in number compared with the group one as shown in Figure 6B.
Fig. 6A. Smaller endoneural congested blood vessels (↑) H&E 
Fig. 6B. Multiple dilated congested blood vessels in the endoneurium (↑) surrounded by inflammatory cell infiltrate (*) H&E X400 
Masson Trichrome stain (collagen deposition and fibrosis): In group one, there was marked increase in thickness (collagen deposition and fibrosis) of epineurium, perineurium and endoneurium as shown in Figure 7A. In group two, there was marked decrease of epineurial and perineurial thickness (fibrosis) compared with group one as shown in Figure 7B.
Fig. 7A. In group one there was marked increase in collagen deposition (fibrosis) in the epineurium (E), perineurium (P) and endoneurium (En). Masson Trichrome X 100 
Fig. 7B. In group two there was marked decrease in epineural (E) and perineural fibrosis (P) compared with group one. Masson Trichrome X100 
Morphometric and statistical analysis
Epineural thickness: Measurement of epineural thickness, which is an indicator of collagen deposition and fibrosis, showed that group one had the highest mean value with significant increase compared with control group and group two. Group two had also significant increase compared with the control, as shown in Figure 8.
3. Mean epineural thickness of group one, group two and control group 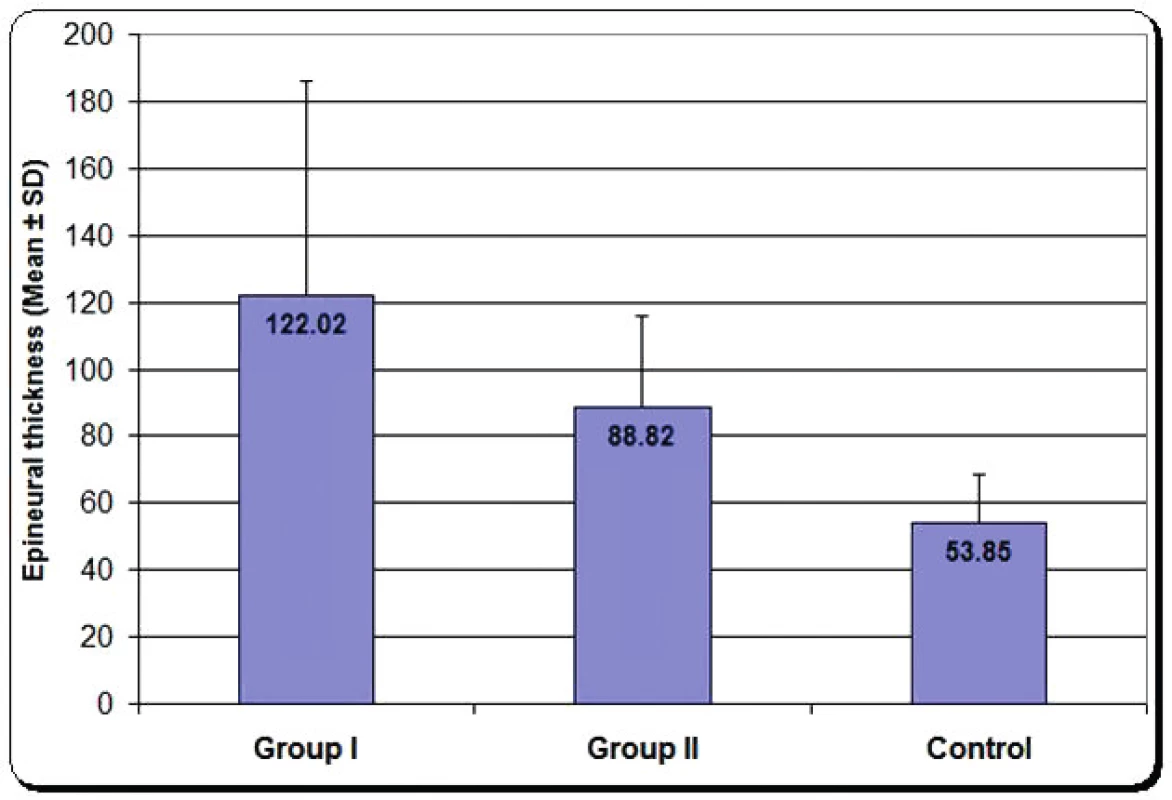
Cross-sectional diameter: Group two had the highest value with significant increase compared with both group one and control group, as shown in Figure 9.
4. Mean cross sectional diameter of group one, group two and control group 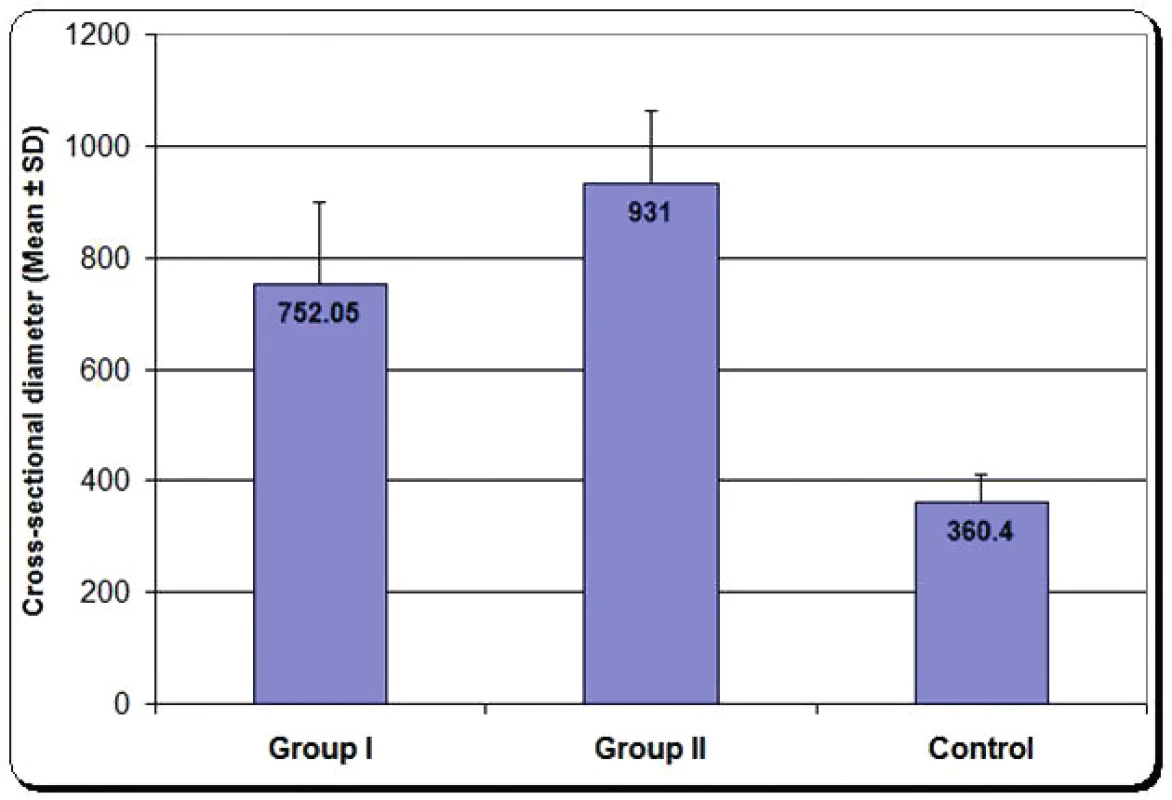
Scar tissue formation index: It is the ratio of epineural thickness divided by the whole cross sectional diameter of the nerve. There was significant decrease in group two compared with group one, as shown in Figure 10.
5. Mean scar tissue formation index of group one, group two and control group 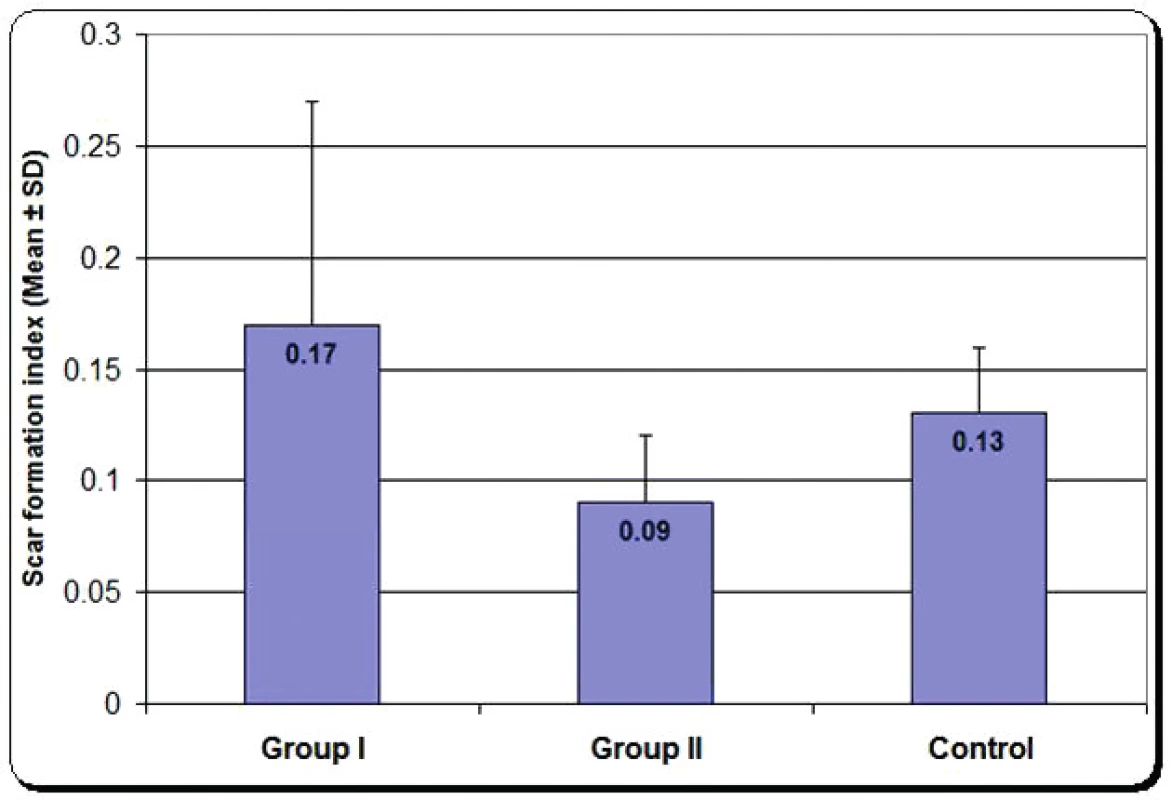
DISCUSSION
In 2005 Martins and his co-workers performed a similar study but with three groups; group one repair performed with suture only, group two repair performed with fibrin glue and group three repair performed with glue and suture. They showed that combination of the two factors had led to poorer regeneration of the nerve7. In our study we operated only on two groups in order to eliminate the factor of the suture and to identify the histological effect of each method alone.
Suture placement has been thought to cause hindrance to the sprouting axons and compression of the blood supply to the fascicles, thereby impairing the regeneration of the transected nerve ends after repair8. Moreover, formation of suture granuloma obstructs myelin and axonal regeneration. Many studies demonstrated inflammation and suture granuloma formation with subsequent neuroma formation after micro-suture repair, which results in focal hindrance to the regeneration of myelin and axons. In this work, we approved the previously mentioned changes but we demonstrated that there is no correlation between the number of sutures and the proper alignment of the nerve fascicles, as with two sutures only at 0 and 180 degrees, there were continuous nerve fibres and fascicles at histological examination. On the other hand, with three or four sutures, there was more tissue handling with subsequent more axonal degeneration.
Histological evaluation of our experiment showed a better outcome of axonal regeneration across the anastomotic site with fibrin glue repair in comparison with suture repair. Sciatic nerve after anastomosis by fibrin glue showed better nerve fibre organization and minimal Schwann cell proliferation compared with that repaired by sutures. Also, the perineural fibrosis, oedema and axonal degeneration in sciatic nerve repaired by fibrin glue were minimal compared with sciatic nerve repaired by suture. Multinucleated giant cells were seen in the suture group, which were not present in the fibrin glue group, which predisposes to neuroma formation. According to Anderson et al. in 2008, the foreign body giant cells (FBGC) are most commonly observed at the tissue/material interface of implanted medical devices, prostheses and biomaterials9.
Moreover, the histological sections showed that the blood vessels in the fibrin glue group were significantly dilated and congested and increased in numbers compared to suture group where the blood vessels were apparently smaller and less in number. This confirms the fact of compressing the blood vessels in anastomosis by micro-sutures, which lead to decreased blood supply to the fascicles. On the other hand, the fibrin glue causes significant capillary and endothelial proliferation at the fibrin glue-treated sites when compared with the controls, which is an important factor in rapid healing of the affected nerve. This was inconsistent with a study performed by Akosy et al., 200910.
In our work, we preferred to apply the fibrin glue in its concerned group of experiments without having any stay supporting stitches to have the best histological assessment and the evaluation of only one methodology used for each group. In addition to confirming the better quality technique for nerve repair by fibrin glue to replace suture technique, our goal was to prove the use of scar tissue formation index as early, simple quantitative measure for recovery of sciatic nerve after anastomosis. We found it easy and it does not require sophisticated methods. Scar tissue formation index is important for normal wound healing but in many clinical situations, scarring interferes with growth, causes deformities, and impairs normal function11. The current study revealed that there was significant decrease in the value of scar tissue formation index of sciatic nerve repaired by fibrin glue.
Operation time is one of the important factors in the choice of the surgical technique in nerve repair. Our results were in agreement with the conclusion of Breshah et al. in 201312, who reported that the application of both n-butyl-2-cyanoacrylate and fibrin glues was easier, simpler and less time consuming. Fibrin glue was superior to the n-butyl-2-cyanoacrylate both functionally and histologically and could be used as an alternative technique to epineural suturing in the microsurgical repair of the transected nerves. In our study the time required to complete nerve anastomosis with fibrin glue technique was significantly shorter than that with suture technique (p<0.05). With the use of fibrin glue, anastomosis time required was 1.85 ± 0.52 minutes. With suture technique, the time was 12.2 ± 2.85 minutes with gradual decrease in time required for anastomosis during the study.
The ideal surgical repair technique should accomplish good wound healing with minimal scar formation and direct the nerve sprouts into their correct targets. Although the scar formation with fibrin glue technique was less than with suture technique, current surgical methods provide variable and often unsatisfactory outcomes. This may result in part because of our inability to stop the scaring process. The development of new therapeutic agents that can reduce scar formation may be the ultimate solution to this problem.
We recommend trying to use fibrin glue in controlled human studies in patients with peripheral nerve injuries. It is possible to expect promising results, similarly as in our study, mainly lower inflammatory reaction, less scaring, shorter time needed for treatment and generally better outcomes.
CONCLUSION
In microsurgical nerve repair suture placement has been thought to cause hindrance to the sprouting axons and compress the blood supply to the fascicles, thereby impairing the regeneration of the transected nerve ends after repair. Moreover, there is a possibility of formation of suture granuloma that obstructs myelin and axonal regeneration. Nerve anastomosis by fibrin glue is a simple, effective technique, which is less time consuming than suturing. Another advantage of this suture-free technique is that it avoids injuring the axon with needles, and the lack of foreign bodies minimizes the inflammatory reaction.
Corresponding author:
Mohamed Elshazly, MBBCh, MSc, M.D.
Plastic Surgery Department,
Assiut University Hospitals
71526 Assiut, Egypt
E-mail: elshazly@aun.edu.eg
Sources
1. West CA, Davies KA, Hart AM, Wiberg M, Williams SR, Terenghi G. Volumetric magnetic resonance imaging of dorsal root ganglia for the objective quantitative assessment of neuron death after peripheral nerve injury. Exp Neurol. 2007 Jan;203(1):22-33.
2. Siemionow M. and Brezicki (2009). Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol; 87, 141-172.
3. Suri A, Mehta VS, Sarkar C. Microneural anastomosis with fibrin glue : an experimental study. Neurol India. 2002 Mar;50(1):23-6.
4. Martins RS, Siqueira MG, Da Silva CF, Plese JP. Overall assessment of regeneration in peripheral nerve lesion repair using fibrin glue, suture, or a combination of the 2 techniques in a rat model. Which is the ideal choice? Surg Neurol. 2005;64 Suppl 1:S1 : 10-6; discussion S1 : 16.
5. Kushawaha, S., et al. Effect of different anaesthetic agents on cardiovascular parameters in male Wistar rats. RJPBCS 2.2 (2011): 685-690.
6. Albayrak BS, Ismailoglu O, Ilbay K, Yaka U, Tanriover G, Gorgulu A, Demir N. Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: outcome based on gross postsurgical, histopathological, and ultrastructural findings. J Neurosurg Spine. 2010 Mar;12(3):327-33.
7. Martins RS, Siqueira MG, Da Silva CF, Plese JP. Overall assessment of regeneration in peripheral nerve lesion repair using fibrin glue, suture, or a combination of the 2 techniques in a rat model. Which is the ideal choice? Surg Neurol. 2005;64 Suppl 1:S1 : 10-6; discussion S1 : 16.
8. Smahel J, Meyer VE, Bachem U. Glueing of peripheral nerves with fibrin: experimental studies. J Reconstr Microsurg. 1987 Apr;3(3):211-20.
9. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008 Apr;20(2):86-100.
10. Aksoy H. et al. Evaluation of the effect of fibrin glue prepared from single-donor plasma on wound healing in rats. Hacettepe University Journal of the Faculty of Pharmacy 29 (2009): 83-93.
11. O’Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol. 1997 Jan;29(1):63-78.
12. Breshah MN, Sadakah AA, Eldrieny EA, Saad KA. Functional and histological evaluation of rat sciatic nerve anastomosis using cyanoacrylate and fibrin glue. Tanta Dental Journal. 2013 August;10(2):67-74.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2017 Issue 2-
All articles in this issue
- Editorial
- COMPLICATIONS OF LOWER EXTREMITY HEMATOMAS IN PATIENTS WITH PRE-INJURY WARFARINE USE
- SURGICAL CORRECTION OF LABIA MINORA HYPERTROPHY, A PERSONAL TECHNIQUE
- SUTURE VERSUS FIBRIN GLUE MICRONEURAL ANASTOMOSIS OF THE FEMORAL NERVE IN SPRAGUE DEWLY RAT MODEL. A COMPARATIVE EXPERIMENTAL ASSESSMENT OF THE CLINICAL, HISTOLOGICAL AND STATISTICAL FEATURES
- INTRAOPERATIVE FAT GRAFTING INTO THE PECTORALIS AND LATISSIMUS DORSI MUSCLES-NOVEL MODIFICATION OF AUTOLOGOUS BREAST RECONSTRUCTION WITH EXTENDED LATISSIMUS DORSI FLAP
- HAS A GLOMUS TUMOR ALWAYS A QUICK DIAGNOSIS?
- CURRENT CONCEPTS IN PERIPHERAL NERVE INJURY REPAIR
- SUBACUTE ARTERIAL BLEEDING AFTER SIMULTANEOUS MASTOPEXY AND BREAST AUGMENTATION WITH IMPLANTS: CASE REPORT
- AUTOLOGOUS FAT TRANSFER, BREAST LIPOMODELLING AND FAT TRANSFER TO THE FACE: CURRENT GOLD STANDARDS AND EMERGING NEW DATA
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- SURGICAL CORRECTION OF LABIA MINORA HYPERTROPHY, A PERSONAL TECHNIQUE
- INTRAOPERATIVE FAT GRAFTING INTO THE PECTORALIS AND LATISSIMUS DORSI MUSCLES-NOVEL MODIFICATION OF AUTOLOGOUS BREAST RECONSTRUCTION WITH EXTENDED LATISSIMUS DORSI FLAP
- COMPLICATIONS OF LOWER EXTREMITY HEMATOMAS IN PATIENTS WITH PRE-INJURY WARFARINE USE
- HAS A GLOMUS TUMOR ALWAYS A QUICK DIAGNOSIS?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career