-
Medical journals
- Career
CURRENT CONCEPTS IN PERIPHERAL NERVE INJURY REPAIR
Authors: R. Kaiser 1; G. Ullas 2; P. Havránek 3; H. Homolková 3; J. Miletín 4; P. Tichá 4; A. Sukop 4
Authors‘ workplace: Department of Neurosurgery and Neurooncology, 1st Faculty of Medicine, Charles University, Military University Hospital Prague, Czech Republic 1; Department of Plastic Surgery, Sandwell and West Birmingham NHS Trust, UK 2; Department of Paediatric and Trauma Surgery, 3rd Faculty of Medicine, Charles University, Thomayer Hospital, Prague, Czech Republic 3; Department of Plastic Surgery, 3rd Faculty of Medicine, Charles University, Hospital Královské Vinohrady, Prague, Czech Republic 4
Published in: ACTA CHIRURGIAE PLASTICAE, 59, 2, 2017, pp. 85-91
INTRODUCTION
Injuries of the peripheral nerves are not rare. They affect 2.8% of trauma patients and result in considerable long-term disability, especially in hand trauma patients.1 They are typically caused by cut or stub wounds in the forearm; lacerations or gunshots are rarer. Neurotrauma needs to be ruled out in every patient with an open limb trauma and at least a basic neurological examination needs to be performed. In case of a suspicion of nerve injury, it is necessary to refer the patient to a specialized centre for revision with possible urgent microsurgical reconstruction.2 Traction injuries may occur in case of low energy trauma (lesion of peroneal nerve in the knee or radial nerve in case of humerus fracture),3 as well as high energy trauma (brachial plexus palsy),4, 5 where the nerve trauma is a typical part of a polytrauma.6 Complete recovery is infrequent and usually limited to relatively minor injuries and reflects neurapraxia and axonothmesis. Laceration of the nerve has no chance of spontaneous recovery and the discontinuity has to be repaired.1
Despite good general knowledge of these injuries, the patients are often referred late for treatment. The aim of this review is to provide information about the basics of nerve anatomy and the pathophysiology of peripheral nerve injuries, as well as potential surgical interventions.
NERVE MICROANATOMY
The nerve fibre consists of an axon and associated Schwann cells. The diameter of an axon is 0.5–20 µm. Myelin sheath covers the axon except of a short segment after exiting from the neuron and at the terminal branching. Schwann cells create the multilayer myelin sheath of myelinated axons by wrapping around the fibre several times during its development. At the site of contact of individual Schwann cells there are areas without myelin cover, so called nodes of Ranvier. The area between two nodes is called an internodal segment, which is longer with thicker fibres.7 The node of Ranvier is also the area where collateral sprouting of axon occurs.8 Non-myelinated fibres have a cover consisting only of Schwann cell folds, which thereby simultaneously “cover” more axons. Apart from production of myelin sheath of the axon, Schwann cells are also a source of growth factors necessary for survival as well as for regeneration of nerve fibres. In case of an injury, neurotrophins are produced in the distal as well as proximal stump and create a suitable environment for re-inervation.9, 10
Nerve fibres are not parallel with the surface of the nerve, but they are undulated. This phenomenon is visible macroscopically as so called bands of Fontana11 and it is one of the basic prerequisites for successful microsurgical suture, since the stumps of the nerve may be pulled back towards each other in a limited distance even after their retraction. Due to the curved arrangement are the nerve fibres up to 20% longer than the actual nerve and therefore it is possible to achieve connection without tension even after short stretching of the nerve.12 Bands of Fontana are not present in the intracranial segment of cranial nerves or in the spinal roots. Banding has also its physiological significance since it enables stretching of the nerves during joint movements. The basis for undulation is a specific structure of endoneurium, which covers the nerve fibre.13
Nerve fibres are organized into fascicles. This arrangement plays an important role in surgical techniques treating nerve injuries. Fascicles are wrapped with perineurium and the whole nerve is separated from the surrounding tissues by epineurium, which is a vascularized tissue that proceeds to mesoneurium, i.e. supportive connective tissue at the site of entry of the vascular bundles.14 Regarding surgery, it is important to maintain the vascular supply of the nerve, which usually originates from a concomitant artery. Many supplying arteries with a diameter of 0.5–1 mm grow distally and their length is 5–15 mm, occasionally even 25 mm. The nerve may be therefore mobilized without their injury only for a short distance. In case of damage to the nutritive vessels (e.g. in the ischiadic nerve in the gluteal area or in the median nerve in the proximal forearm) there exists a possibility of nerve ischemia. Quantity of fibrous tissue grows in the area around the joint and in the nerves with greater quantity of small fascicles. Number of fascicles is significantly variable in various nerves and ranges from one to a hundred, while each can contain up to 10 thousand axons.14 The structure of the nerve in a section in the proximal segments is significantly disorganized and organization increases with more distal localisation. This phenomenon significantly complicates surgery of proximal injuries, while it is sometimes very difficult to impossible to determine corresponding fascicles in case of injuries with loss of tissues.15
TYPES OF NERVE INJURIES
Traction injuries occur when the elastic capacity of the nerve that is based on collagenous endoneurium is exceeded. These are typical for injuries of the brachial plexus; they are frequent at the level of the knee with the injury of the peroneal nerve or in case of fractures, typically fracture of the humerus with the injury of the radial nerve. In lacerations, the nerve may be completely interrupted, however, more common is an injury with interruption in the continuity with partial division of the nerve diameter. They comprise up to 30% of all nerve injuries.12 Nerves may also be injured by compression, e.g. so called “Saturday night palsy“ of the radial nerve (falling asleep with arm leaning on an edge of a bar) or entrapment syndromes (nerve compression). Pathophysiology is not completely explained; there is a possibility of complete loss of sensory and motoric functions. Lesions are attributed to a combination of compression with ischemia, however it is not clear, which of the two is predominant. Histologically there are no obvious changes and injury is reversible, if ischemia lasts less than 8 hours.16
The process of changes that occur after nerve injury was first described by Waller in 1850,17 while the conclusions about degeneration and subsequent regeneration of the distal stump (and a small segment of proximal stump) are valid till now. The basic Seddon classification 18 to neurapraxia (‘concussion’ of the nerve, temporary loss of conductive function), axonotmesis (interruption of axons without injury to mesenchymal parts) and neurotmesis (interruption of a nerve) was subsequently extended by Sunderland 14 to five degrees – injury to myelin, axon, endoneurium, perineurium and epineurium.
NERVE DEGENERATION
Distal segment
In neurapraxia there are no histological changes; functionally it is only a conduction block. In axonotmesis, there may be various morphological changes. This can be Waller degeneration, which starts within several hours after the injury and is based on fragmentation of axon and myelin distally from the site of injury. There is loss of axonal continuity with loss of conduction in axon within 48-96 hours after the injury. Decay of myelin occurs between 36 and 48 hours. Schwann cells play a key role in this whole process. The degradation process ends in 5 to 8 weeks and its result is presence of nerve fibre residues consisting of endoneurium filled with Schwann cells. These changes are more apparent in case of neurotmesis. After interruption of the fibres, retraction occurs due to the effect of elastic endoneurium, swelling at the site of interruption and haemorrhage resulting in local inflammatory response. Activation of fibroblasts leads to scaring on the ends of the nerves as well as between the fascicles. The stumps are therefore thicker than a healthy nerve; the whole area is also fixed with a scar to the surrounding tissue. In case of the 4th and 5th degree, there are - apart from obvious motoric and sensitivity disorders in the appropriate nerve supply area - also vasomotor and apocrine dysfunction due to interruption of efferent sympathetic fibres with the development of red dry skin in the area without innervation. 2
Apart from the periphery, there is also the central part affected. In the first phase there is significant proteosynthesis as a preparation of the neuron to repair the defect. If there is no conductive structure present in the distal stump with appropriate microenvironment, there is a great amount of grow cones growing from the axon (50–100), which together with Schwann cells and connective tissue form a neuroma. In case of opposite situation, there are several grow cones, which grow to the distal stump.19, 20 Columns of Schwann cells are called bands of Büngner. Their main function is guidance and support of the newly growing axon by the presence of adhesive molecules and enzymatic activity. Schwann cells are also able to migrate in a limited extent to the space between the distal and proximal stump of an interrupted nerve. They create some kind of bridge together with fibroblasts and fibrin matrix, which participates during navigation of growing axons. In case of unsuccessful reinervation, Schwann cells undergo regressive changes and Büngner columns become atrophic and their number is significantly reduced within several months.21
In case of 4th to 5th degree of injury, the situation is more complicated. The continuity of the axon as well as connective parts of the nerve is impaired. Interrupted ends are significantly macroscopically changed; they create an oedematous mass of disorganized Schwann cells, capillaries, fibroblasts, macrophages and collagen. Regenerating axons are usually stopped by a new scar already before they reach the end of the proximal stump, some of them grow through the scar to the surrounding tissue and part of them change the direction of growth and grow back to the proximal stump. Small part of them can reach the distal stump, as long as the gap in-between is not too wide, but even then, there is a new scar that prevents further growth.22
Proximal segment
The extent of changes in neurons and fibres proximally from the injury depends on the extent of the injury and distance from the cellular body. In a small distance from the site of injury, there is degeneration of Schwann cells with reduction of myelin and thickness of the axon. These changes could be only minimal, extending to the first Node of Ranvier or it could affect the whole length up to the cellular body. The second option occurs if there is also apoptosis of neuron associated with the injury. The whole proximal segment then undergoes Wallerian degeneration and it is subject to phagocytosis.23
The damaged neuron is then subject to changes within the first six hours after the injury. Firstly chromatolysis occurs. The change corresponds to increased metabolic activity of neuron during axonal regeneration. Proteosynthesis continues in the case of a nerve suture, when the proximal stump is near to the Schwann cells of the distal stump that produce growth factors. If there is no reconstruction performed, neurons undergo gradual atrophy and this process ends with their extinction. The total quantity of dead neurons is not known. For example apoptosis of cells in a spinal ganglion after axonotmesis affects 20 – 50% of neurons.23
NERVE REGENERATION
Wallerian regeneration in case of severe injuries starts only after a previous degeneration. Human peripheral neurons have a capacity to start sufficiently strong regeneration within a period up to 12 months after an injury and massive response is possible also after repeated injury. In the cases of neurapraxia and axonotmesis, there is always functional restoration achieved. This occurs frequently after overcoming the conduction block, or later after axonal reparation. Injuries do not leave any significant functional or morphological consequences.14
In cases of more severe injuries with impaired endoneurium, there is a loss of axonal support for their distal growth. They grow partially to the surrounding tissues or to non-adequate endoneural tubes. Functional result is therefore significantly limited and it is dependent on the severity of the impairment. More severe injuries are associated with scars. Axons must first find a way to a newly constituted or distally preserved endoneural tube. The distal stump may contain more axons at the end of reparation than the proximal stump due to collateral division.21
Generally accepted average speed of axonal growth is 1 mm per day, although it can reach 0.5 to 9 mm daily. These differences are due to several variables. Regeneration speed declines with increasing distance from the neuron and higher age. Differences also exist between sensory and motoric axons. The growing axon is often associated with the presence of a positive Tinel´s sign, i.e. pain on percussion over the area of current position of axonal cone.16, 22
Axonal outgrowth and regeneration across and into the distal nerve stump may be slowed down by specific inhibitory molecules, especially some types of glycoproteins.24
The studies have shown that advanced age correlates with poor prognosis even after technically successful nerve repair. Schwann cells in the aged animal pose a primary impediment to axon regeneration in older animals as they fail to support regenerating axons.1
CHANGES IN TARGET ORGANS
In case of denervation, the target organs undergo characteristic changes. These are progressive atrophy (more than 60% of mass and 90% of maximal strength during the first six months after denervation) and subsequent fibrosis of muscles (occurring in a variably long time, usually within two years after denervation). These changes are dependent on the speed at which regeneration of the neuro-muscular connection occurs. This time consists of not just the duration of actual nerve regeneration after reconstruction, but also latency between injury and operation. Prolonged division of an axon, which takes more than six months before repair, results in up to two-third reduction of regenerating motoric axons.16, 25 Immobilisation inducing degenerative changes of the joints and adjacent structures (i.e. ligaments and tendons) could contribute to the worse result.26
ACCELERATION OF RE-INERVATION DURING SURGICAL THERAPY
It has been recognized, in general, that the time frame between the injury and re-inervation (i.e. successful connection of regenerated axons to the motoric endplates) should not be longer than 24 months, which in case of a lesion of the lower part of brachial plexus virtually excludes the possibility of re-inervation of small muscles in the hand and it also makes re-inervation of the forearm muscles more difficult. This does not apply, however, to the cranial nerves, while for example in the facial nerve it is possible to achieve re-inervation in some cases even several years after the injury.27 This problem can be solved either by acceleration or support of axonal growth (using trophic factors, currently only in an experimental phase) 28, 29 or by connection of the source axons closer to the target muscle using a donor nerve, i.e. nerve transfer. This changes the proximal lesion to a distal one.30
Speed of regeneration may be effectively influenced by electrical stimulation. A brief period of electrical stimulation of 1 hour was shown to be as effective as continual stimulation for promoting both motor and sensory regeneration. It mediates its effect at the level of axonotomized neurons, dominantly by upregulation of BDNF and its trkB receptors followed by upregulation of cytoskeletal proteins and GAP-43 in motor neurons.24
TIMING OF SURGICAL PROCEDURE
According to the type of injury, it is possible to use the rule 3 x 3 in practice: 2
- Immediately, or within 3 days – sharp clean injuries (cut or stab wounds), where finding of the distal stump may be facilitated by electrostimulation (distal segment is conductive for up to 72 hours after interruption). Every injury is an acute condition. It is therefore advisable to perform immediate revision, which usually enables suture without the use of grafts.
- Within 3 weeks – lacerated dirty wounds (bites, extensive lacerations spoiled with e.g. soil, gunshot wounds, large vascular reconstructions) after primary treatment with antibiotics and cleaning of the wound, suture of tendons, muscles and vessels. If there is the end of the nerve found, it is suitable to mark it with Silon suture. Within 3 weeks after the injury, destructive changes begin on the ends of the stumps with the development of the so called terminal neuroma. Delayed surgery is performed to define the scope of nerve injury and to achieve sufficient resection of neuroma, which, if left in place, may lead to unsuccessful regeneration. After cutting it with a “salami” technique it is sure that healthy-appearing nerve tissue will not be changed anymore.
- In 3 to 6 months – all closed injuries after EMG examination demonstrating persisting complete denervation syndrome of a particular nerve. In cases of isolated functional impairment (neurapraxia), the function starts to restore in about 3 weeks. In case of axonotmesis, it is possible to expect at least electrophysiological changes of re-inervation in 2–3 months. Their absence suggests either compression of a nerve by a scar or neurotmesis (i.e. neuroma in continuity or nerve rupture).31
NEUROLYSIS
In cases of blunt closed injuries, there is a possibility that during surgery it is found that the affected nerve segment – the so called neuroma in continuity - is still conductive, i.e. it is possible to trigger nerve action potential (NAP positive). In these injuries, it is indicated to perform simple nerve release, i.e. neurolysis. Currently, there is mostly exoneurolysis performed, i.e. dissection of the nerve from the surrounding scar tissue, which is possibly supplemented with epineurotomy. Endoneurolysis, i.e. dissection of the nerve to individual fascicles has no justification at present. On the contrary, there is a risk of subsequent excessive intramural scaring with deterioration of the condition.32
If the affected neuroma is not conductive (NAP negative), there is always a scar palpable and usually also grey-violet colour of a particular segment. This segment is removed at the assumed border and both ends are cut with gradual salami technique up to macroscopically healthy tissue with visible fascicles. Then, one of the following reconstructive techniques is used.12, 14, 18
RECONSTRUCTIVE TECHNIQUES
Nerve reconstruction should follow some basic principles. It is necessary to perform suture under magnification by using a microscope or loupe glasses. Nerve stumps have to be adequately oriented by observation of vascular pattern and diameter of each fascicle and sutured by 8–0 to 10–0 sutures without tension. 2, 12
Two basic techniques of nerve suture are used. Based on long term experience and large groups, it is not possible to definitively state, which technique is better. The result depends on several variables – time from injury, the age of the patient, mechanism of the trauma, type of nerve, composition of the fibres in the nerve (pure motoric and sensitive or mixed) and localization of the injury.2 Proximal injury is worse due to two reasons: 1. longer re-inervation route, 2. fascicles, mainly those intended for distal structures, are mixed and in their course are mutually connected. Each motoric and sensitive fascicle is well formed up to the distal area.
Last but not least, the result is influenced by the length of the defect between the stumps (tension, usage of a graft) or simultaneous vascular injury with ischemia.32
Epineural suture – the nerve is sutured with microsurgical technique to epineurium after approximation of both ends and microsurgical removal of its external layer usually by 8-0 suture.32
Fascicular (perineural) suture – performed in perineurium or in interfascicular epineurium with greater microscope magnification. The method enables better coaptation of fascicles; it is advantageous in distal injuries, i.e. in areas with several well-differentiated large fascicles. It is used mainly as group fascicular technique, during which are mutually connected certain groups of corresponding fascicles in the nerve. It is advisable to use fine monofilament sutures (usually 10-0).12 However, the technique is associated with greater trauma and scaring to the healing nerve internally due to presence of permanent sutures. Therefore, fascicular repair has no functional superiority over epineural suture.1
Adhesion with fibrin glue – method originally developed to prevent scaring associated with each suture and acceleration of surgical procedures. Use of fibrin glue may be associated with the occurrence of dehiscence and for stability of the connection it is possible to use one or two stitches.33, 34 Experimental studies have shown that the use of glue results in less granulomatous reaction at the site of the suture and better axonal regeneration. The changes are not very significant,35 and moreover there was no controlled human study performed to confirm the differences between the methods.36
In every injury, even in a clean cut, there is a defect between the stumps based on nerve retraction.37 In case of more severe or late treated injuries is retraction greater due to intramural fibrosis, and of course there is a defect in case of a possible injury with soft tissue loss. To achieve suture without tension, it is necessary to eliminate the defect or replace it:
Techniques that enable shortening of the defect
Stretching of the stumps – this is enabled by the undulated course of the nerve fibres (bands of Fontana). It is possible to stretch only the stumps in fresh clean sharp injuries, where retraction is minimal (1–2 cm). If stretching is more than 5% of the length of the nerve, there is reduced blood perfusion in vasa nervorum of the nerve and in cases more than 15%, the perfusion stops.38
Mobilisation of the nerve – cutting of the supportive mesoneurium and release of the nerve from the surrounding tissues. The disadvantage is reduced vascular supply of the particular segment, therefore the released segment should not be longer than 6 to 8 cm. Mobilisation enables shortening of the defect by 2–4 cm.39
Transposition – in case of nerve mobilisation supplemented with a shift to a position, which is more in the line with further course of the nerve. This is used typically in ulnar nerve injury in the elbow, when its transfer over the medial epicondyle enables shortening of the defect by 3–5 cm. The method may be used in the radial nerve, which may be moved ventrally over the humerus with a gain of up to 3 cm.12
In case of bigger defects, none of the aforementioned methods can shorten the distance between the stumps and for the success of the procedure it is necessary to use a graft to bridge the gap. The great results of Millesi 40 and Samii 41 in the 70’s and 80’s of the 20th century contributed to a wider use.
Bridging of the defect with grafts
In cases where it is impossible to achieve tension-free suture of nerve stumps, the gap between them must be bridged. Use of an autograft remains the most reliable method. Three conditions must be fulfilled for the best result during the use of a graft:
- The graft should cover the whole diameter of the affected nerve
- It is commonly advised to choose a graft that is 10–20% longer than the gap to ensure a tension-free suture
- The nerve must be well nourished.42 Diffusion to the central areas of the graft can be ensured only in thin nerves.31 The critical diameter of the graft is 2 to 5 mm.41 This condition may be fulfilled with the use of several grafts with a smaller diameter. With the increasing number of grafts, declines diffusion capacity. It has been shown however, that with the use of two anastomoses while avoiding tension in case of the graft, are achieved better results than in case of one anastomosis without a graft under tension.40-42
In case of oligofascicular nerves, it is possible to use interfascicular connection. In larger nerves it is, however, currently the preferred cable technique. This is when the grafts are sutured between the stumps of the nerve to cover most or whole diameter of the affected nerve.43 The most frequently used donor remains the sural nerve. In case of surgeries on the upper limb, it is possible to use the lateral cutaneous nerve of the forearm. Generally, it is possible to achieve restoration of function in grafted great nerves (median and ulnar nerve) in about 60% of cases. Better effect may be expected in the sensory component of the nerve.44
It has been well documented that nerves might regenerate across a short nerve gap through various conduits, such as veins, pseudosheats or bioabsorbable tubes. Vein grafts have been successfully used to reconstruct distal sensory nerve defects.1 However, there was no alternative found yet, which would have the same good regenerative capacity as a nerve graft in an experiment.45
Nerve transfers
Nerve transfer is a method that uses an intact, functionally less important donor nerve as a source of axons for the injured nerve in cases when the proximal stump cannot be used for repair (avulsion of cervical roots or severe injury of the proximal stump with a loss of tissue). The donor is interrupted and its proximal stump is sutured to the distal stump of the reconstructed nerve (recipient). The benefit of this technique is only one neurorrhaphy site (contrary to graft repair) shortening of regeneration time because it minimizes the distance over which a nerve has to regenerate.25, 46 The technique is typically used in brachial plexus injuries. It is possible to use intraplexal (from the plexus – e.g. pectoral nerve, thoracodorsal nerve, etc.) and extraplexal nerves (spinal accessory nerve, intercostal nerves, phrenic nerve) for neurorrhaphy, whereas the loss of function of a certain muscle while sacrificing its nerve must always be outweighed by the expected result. Recently, there has been popular reconstruction of proximal ulnar nerve injuries with a transfer of the distal end of the anterior interosseus nerve (from median nerve) to the deep branch of the ulnar nerve 47, 48 or injuries to the median nerve by suturing the same nerve to its recurrent branch at the level of the wrist.48, 49 Apart from the brachial plexus, there were also described nerve reconstructions using surrounding donors on the lower limbs. These are however isolated case reports only.2
In certain cases it is possible to find a compromise solution, i.e. partial preservation of function and also reinervation of the recipient. Apart from the popular technique with the use of one of the branches of the radial nerve for the triceps muscle for reinervation of axillary nerve it is a fascicular transfer and end-to-side neurorrhaphy. 50
Fascicular transfer was firstly described by Oberlin in 1994 (hence so called Oberlin’s technique) as a transfer of motoric fascicle of the ulnar nerve for the flexor carpi ulnaris muscle to the musculocutaneus nerve.51 Later was described similar procedure in the median nerve with harvesting of a fascicle for pronator teres muscle and for flexor carpi radialis muscle.52 The technique has generally very good results and it can be used in cases of injuries of the upper roots of the brachial plexus. The fascicle, which is found with electric stimulation after opening epineurium of a particular nerve, is subsequently divided and its proximal stump is released in a length of several centimetres. This stump is then sutured to the distal stump of the reconstructed nerve.53 Due to the short reinervation route this technique is usable in older injuries, which are treated in more than one year after the injury.54 Recently, this technique has been used with good results in the reconstruction of axillary nerve.55
End-to-side neurorrhaphy of peripheral nerves is based on the fact that apart from the terminal branches, the axons are also capable of dividing within their course. This is termed collateral sprouting. The principle of the technique is connection of the distal stump of the affected nerve to the side of an intact donor nerve after the creation of a perineural window.56 The technique is mainly used in reconstruction of digital nerves.57 It has very limited effect on greater nerves.58
INJURY TO THE PERIPHERAL NERVES IN CHILDREN
Lesions of the peripheral nerves in children are less common in comparison with the adult population. More frequently occurring are peripheral nerve injuries in association with fractures of the limbs. Closed injuries predominate and among these fractures most common are supracondylar fractures. Neural lesions are almost exclusively of neurapraxia or axonotmesis type, interruption of anatomical continuity of the nerve stem is very rare. Treatment of these blunt injuries is conservative, even in case of a full denervation syndrome it is possible to wait for several months. Open injuries of peripheral nerves in childhood occur by cutting, mostly by glass from windows or doors and dishes.
Consequences of peripheral nerve injuries with regards to pathophysiology are the same as in adult patients. The results of healing depend on the age of the child. Neuronal activity of injured peripheral nerves restores very quickly in very small children, axonal regeneration occurs with a rate up to 5 mm per day and the results of healing of injured peripheral nerves in children are therefore better than in adults. This is attributed mostly to neural plasticity of the nerve tissue.59
Timing of surgical revision of injured peripheral nerve in children is the same as in adult patients; also usage of perioperative electrophysiological monitoring is mandatory. The preferred surgical techniques in children that are used are exoneurolysis, anastomosis with a graft, primary anastomosis and removal of neuroma in continuity.
POSTOPERATIVE COURSE
Sutured area reaches the original strength in 2–3 weeks. The limb should therefore be fixed for this period of time. In more complex injuries requiring tendon or muscle repair, the limb must be fixed for longer period of 4–6 weeks. Alternatively, after two weeks of immobilization, a dynamic splint may be used. Then it is necessary to start with intensive daily rehabilitation, electrostimulation of denervated muscles and sensitive stimulation of denervated skin.60 There is also a crucial role for custom-made orthosis applied usually during the night as a prevention of flexion contractures. The patient must be instructed about minimal latency between operations and primary signs of reinervation. This lasts usually around one year; the first electrophysiological signs of reinervation may be sometimes noted six months after the procedure.32
ACHIEVEMENT OF DEFINITIVE STATUS
There is an agreement currently that for evaluation of the definitive status, there is two-year period of observation necessary.32 Muscle strength is most frequently tested with the MRC system (Medical Research Council, Table 1).14 Evaluation of sensitivity is most important for reconstruction of median and tibial nerve. Most commonly used is the scale of Mackinnon and Dellon (Table 2).61
1. Classification of motor recovery according to the MRC scale<sup>14</sup> 
2. Classification of sensory recovery according to the Mackinnon-Dellon scale.<sup>60</sup> s2PD – static sense of two-point discrimination 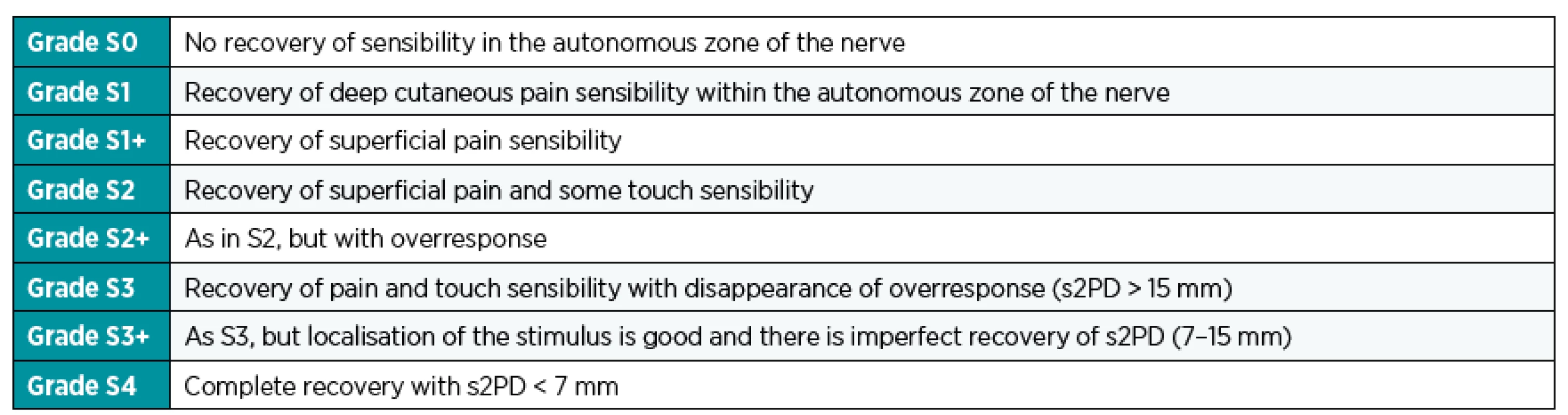
In case of unsuccessful (or functionally ineffective) reinervation of the affected nerve, which occurs in 20–30% of nerve injuries, secondary correction methods may be used for restoration of limb function. These are tendon or muscle transfers and muscle transposition procedures.62
CONCLUSION
Nerve injuries deserve our attention in spite of low incidence due to common serious morbidity caused by denervation of affected muscles and skin. The outcome of surgical therapy is often good. However, such results may be achieved only with timely revision. In case of sharp injuries ideally within 72 hours and in closed lesions between 3 and 6 months from their occurrence, if there are no signs of regeneration. Irritation due to compression after fractures or iatrogenic injuries can be solved even later. The most important request is therefore the timely referral of the patient to a specialized unit where the microsurgical repair, including nerve grafting, is possible.
Corresponding author:
Radek Kaiser, M.D., Ph.D.
Department of Neurosurgery and Neurooncology, 1st Faculty of Medicine, Charles University, Military University Hospital Prague
U Vojenské nemocnice 1200, 169 02 Prague 6
Czech Republic
E-mail: radek.kaiser@uvn.cz
Sources
1. Houschyar KS, Momeni A, Pyles MN, et al. The Role of Current Techniques and Concepts in Peripheral Nerve Repair. Plast Surg Int. 2016;2016 : 4175293.
2. Kaiser R et al. Chirurgie hlavových a periferních nervů s atlasem přístupů. Praha: Grada Publishing; 2016.
3. Kaiser R, Houšťava L, Mencl L, Brzezny R, Haninec P. Treatment of peroneal nerve injury by operation. Cesk Slov Neurol N. 2011;74 : 187-90.
4. Kaiser R, Waldauf P, Haninec P. Types and severity of operated supraclavicular brachial plexus injuries caused by traffic accidents. Acta Neurochir. 2012;154 : 1293-7.
5. Kaiser R, Haninec P. The influence of seatbelts on the types of operated brachial plexus lesions caused by car accidents. J Hand Surg. 2012;37 : 1657-9.
6. Kaiser R, Mencl L, Haninec P. Injuries associated with serious brachial plexus involvement in polytrauma among patients requiring surgical repair. Injury. 2014;45 : 223-6.
7. Raine CS. Differences between the nodes of Ranvier of large and small diameter fibres in the P.N.S. J Neurocytol. 1982;11 : 935-47.
8. Hopkins WG, Brown MC, Keynes RJ. Nerve growth from nodes of Ranvier in inactive muscle. Brain Res. 1981;222 : 125-8.
9. Grafstein B. Cellular mechanisms for recovery from nervous system injury. Surg Neurol. 1980;13 : 363-5.
10. Mackinnon SE, Dellon AL, Lundborg G, Hudson AR, Hunter DA. A study of neurotrophism in a primate model. J Hand Surg. 1986;11 : 888-94.
11. Fontana F. Traité sur le vénin de la vipère, sur les poisons américains, sur le laurier-cerise et sur quelques autres poisons végétaux. On y a joint des observations sur la structure primitive du corps animal. Différentes expériences sur la reproduction des nerfs et la description d’un nouveau canal de l’oeil. Florence. 1781 : 187-221.
12. Zvěřina E, Stejskal L. Poranění periferních nervů. Praha: Avicenum; 1979.
13. Haninec P. Undulating course of nerve fibres and bands of Fontana in peripheral nerves of the rat. Anat Embryol. 1986;174 : 407-11.
14. Sunderland S. Nerves and Nerve Injuries. New York: Churchill Livingstone; 1978.
15. Gruber H. Identification of motor and sensory funiculi in cut nerves and their selective reunion. Br J Plast Surg. 1976;29 : 70-3.
16. Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1.
17. Waller AV. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations on the alterations produced thereby in the structure of their primitive fibres. Philosophical Transactions of the Royal Society of London. 1850 : 423-9.
18. Seddon H. Surgical Disorders of the Peripheral Nerves, 2nd ed. London: Churchill Livingstone; 1972.
19. Gutmann E, Sanders FK. Recovery of fibre numbers and diameters in the regeneration of peripheral nerves. J Physiol. 1943;101 : 489-518.
20. Toft PB, Fugleholm K, Schmalbruch H. Axonal branching following crush lesions of peripheral nerves of rat. Muscle Nerve. 1988;11 : 880-9.
21. Johnson EO, Vekris MD, Zoubos AB, Soucacos PN. Neuroanatomy of the brachial plexus: the missing link in the continuity between the central and peripheral nervous systems. Microsurgery. 2006;26 : 218-29.
22. Kaiser R, Haninec P. Degeneration and regeneration of the peripheral nerve. Cesk Fysiol. 2012;61 : 9-14.
23. Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg. 2000;25 : 391-414.
24. Gordon T. Nerve Regeneration: Understanding Biology and Its Influence on Return of Function After Nerve Transfers. Hand Clin. 2016;32 : 103-17.
25. Brown JM, Shah MN, Mackinnon SE. Distal nerve transfers: a biology-based rationale. Neurosurg Focus. 2009;26:E12.
26. Samii M, Carvalho GA, Nikkhah G, Penkert G. Surgical reconstruction of the musculocutaneous nerve in traumatic brachial plexus injuries. J Neurosurg. 1997;87 : 881-6.
27. Volk GF, Pantel M, Guntinas-Lichius O. Modern concepts in facial nerve reconstruction. Head Face Med. 2010;6 : 25.
28. Kaiser R, Dubový P, Haninec P. Vascular endothelial growth factor. Cesk Fysiol. 2011;60 : 48-51.
29. Haninec P, Kaiser R, Bobek V, Dubový P. Enhancement of musculocutaneous nerve reinnervation after vascular endothelial growth factor (VEGF) gene therapy. BMC Neurosci. 2012;13 : 57.
30. Haninec P, Kaiser R. Surgical treatment of brachial plexus injury. Cesk Slov Neurol N. 2011;74 : 619-30.
31. Lundborg G. Nerve Injury and Repair. New York: Churchill Livingstone; 1988.
32. Kim DH, Kline DG. Kline & Hudson’s nerve injuries: Operative results for major nerve injuries, entrapments and tumors. Philadelphia, PA: Saunders Elsevier; 2008.
33. Moy OJ, Peimer CA, Koniuch MP, Howard C, Zielezny M, Katikaneni PR. Fibrin seal adhesive versus nonabsorbable microsuture in peripheral nerve repair. J Hand Surg. 1988;13 : 273-8.
34. Narakas A. The use of fibrin glue in repair of peripheral nerves. Orthop Clin North Am. 1988;19 : 187-99.
35. Sameš M, Blahoš J, Rokyta R, Beneš V. Comparison of microsurgical suture with fibrin glue connection of the sciatic nerve in rabbits. Physiol Res. 1997;46 : 303-6.
36. Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127 : 2381-90.
37. Daniel RK, Terzis JK. Reconstructive microsurgery. Boston: Litle Brown; 1977.
38. Lundborg G, Rydevik B. Effects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineurium. J Bone Joint Surg Br. 1973;55 : 390-401.
39. Kline DG, Hackett ER, Davis GD, Mayers MB. Effect of mobilisation on the blood supply and regeneration of injured nerves. J Surg Res. 1972;12 : 254-66.
40. Millesi H, Meissl G, Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972;54 : 727-50.
41. Samii M. Modern aspects of peripheral and cranial nerve surgery. Adv Tech Stds Neurosurg. 1975;2 : 33-85.
42. Millesi H. Nerve grafting. Clin Plast Surg. 1984;11 : 105-13.
43. Kaiser R, Ullas G. Acutely reconstructed isolated supraclavicular brachial plexus injury caused by a chainsaw. Plast Surg Case Studies. 2016;2 : 7-8.
44. Yang M, Rawson JL, Zhang EW, Arnold PB, Lineaweaver W, Zhang F. Comparisons of outcomes from repair of median nerve and ulnar nerve defect with nerve graft and tubulization: a meta-analysis. J Reconstr Microsurg. 2011;27 : 451-60.
45. Pettersson J, McGrath A, Kalbermatten DF, et al. Muscle recovery after repair of short and long peripheral nerve gaps using fibrin conduits. Neurosci Lett. 2011;500 : 41-6.
46. Bertelli JA, Ghizoni MF. Concepts of nerve regeneration and repair applied to brachial plexus reconstruction. Microsurgery. 2006;26 : 230-44.
47. Tubbs RS, Custis JW, Salter EG, Blount JP, Oakes WJ, Wellons JC, 3rd. Quantitation of and landmarks for the muscular branches of the ulnar nerve to the forearm for application in peripheral nerve neurotization procedures. J Neurosurg. 2006;104 : 800-3.
48. Sassu P, Libberecht K, Nilsson A. Nerve transfers of the forearm and hand: a review of current indications. Plast Aesthet Res. 2015;2 : 195-201.
49. Vernadakis AJ, Humphreys DB, Mackinnon SE. Distal anterior interosseous nerve in the recurrent motor branch graft for reconstruction of a median nerve neuroma-in-continuity. J Reconstr Microsurg. 2004;20 : 7-11.
50. Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
51. Oberlin C, Beal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg. 1994;19 : 232-7.
52. Songcharoen P, Mahaisavariya B, Wongtrakul S, Lamsam C. Ipsilateral median nerve´s fascicle transfer for restoration of elbow flexion in root avulsion brachial plexus injury. J Thai Orthop Surg. 2001;26 : 93-5.
53. Nath RK, Lyons AB, Bietz G. Physiological and clinical advantages of median nerve fascicle transfer to the musculocutaneous nerve following brachial plexus root avulsion injury. J Neurosurg. 2006;105 : 830-4.
54. Kim DH, Cho YJ, Tiel RL, Kline DG. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98 : 1005-16.
55. Haninec P, Kaiser R. Axillary nerve repair by fascicle transfer from the ulnar or median nerve in upper brachial plexus palsy. J Neurosurg. 2012;117 : 610-4.
56. Haninec P, Kaiser R, Dubový P. A Comparison of collateral sprouting of sensory and motor axons after end-to-side neurorrhaphy with and without the perineurial window. Plast Reconstr Surg. 2012;130 : 609-14.
57. Ogun TC, Ozdemir M, Senaran H, Ustun ME. End-to-side neurorrhaphy as a salvage procedure for irreparable nerve injuries. Technical note. J Neurosurg. 2003;99 : 180-5.
58. Haninec P, Mencl L, Kaiser R. End-to-side neurorrhaphy in brachial plexus reconstruction. J Neurosurg. 2013;119 : 689-94.
59. Tajima T, Imai H. Results of median nerve repair in children. Microsurgery. 1989;10 : 145-6.
60. Humhej I, Sameš M. Poranění periferních nervů u dětí a mladistvých. Čes-slov Pediat 2015;70 : 20-8.
61. Mackinnon SE, Dellon AL. Surgery of the peripheral nerve. New York: Thieme; 1988.
62. Krishnan KG, Martin KD, Schackert G. Traumatic lesions of the brachial plexus: an analysis of outcomes in primary brachial plexus reconstruction and secondary functional arm reanimation. Neurosurgery. 2008;62 : 873-85.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2017 Issue 2-
All articles in this issue
- Editorial
- COMPLICATIONS OF LOWER EXTREMITY HEMATOMAS IN PATIENTS WITH PRE-INJURY WARFARINE USE
- SURGICAL CORRECTION OF LABIA MINORA HYPERTROPHY, A PERSONAL TECHNIQUE
- SUTURE VERSUS FIBRIN GLUE MICRONEURAL ANASTOMOSIS OF THE FEMORAL NERVE IN SPRAGUE DEWLY RAT MODEL. A COMPARATIVE EXPERIMENTAL ASSESSMENT OF THE CLINICAL, HISTOLOGICAL AND STATISTICAL FEATURES
- INTRAOPERATIVE FAT GRAFTING INTO THE PECTORALIS AND LATISSIMUS DORSI MUSCLES-NOVEL MODIFICATION OF AUTOLOGOUS BREAST RECONSTRUCTION WITH EXTENDED LATISSIMUS DORSI FLAP
- HAS A GLOMUS TUMOR ALWAYS A QUICK DIAGNOSIS?
- CURRENT CONCEPTS IN PERIPHERAL NERVE INJURY REPAIR
- SUBACUTE ARTERIAL BLEEDING AFTER SIMULTANEOUS MASTOPEXY AND BREAST AUGMENTATION WITH IMPLANTS: CASE REPORT
- AUTOLOGOUS FAT TRANSFER, BREAST LIPOMODELLING AND FAT TRANSFER TO THE FACE: CURRENT GOLD STANDARDS AND EMERGING NEW DATA
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- SURGICAL CORRECTION OF LABIA MINORA HYPERTROPHY, A PERSONAL TECHNIQUE
- INTRAOPERATIVE FAT GRAFTING INTO THE PECTORALIS AND LATISSIMUS DORSI MUSCLES-NOVEL MODIFICATION OF AUTOLOGOUS BREAST RECONSTRUCTION WITH EXTENDED LATISSIMUS DORSI FLAP
- COMPLICATIONS OF LOWER EXTREMITY HEMATOMAS IN PATIENTS WITH PRE-INJURY WARFARINE USE
- HAS A GLOMUS TUMOR ALWAYS A QUICK DIAGNOSIS?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

