Možnosti zavedení časné nádorové regrese jako potenciálního prediktivního markeru do každodenní klinické praxe u pacientů s metastatickým kolorektálním karcinomem RAS divokého typu léčených cetuximabem – neintervenční observační studie
Feasibility of implementation of the early tumor shrinkage as a potential predictive marker to daily clinical practice in patients with RAS wild type metastatic colorectal cancer, treated with cetuximab – a non-interventional observational study
Background: With the aim to show the feasibility of early tumor shrinkage (ETS) concept implementation into daily clinical practice in the Czech Republic, a non-interventional, multicentric, single arm, prospective study in real world set-up was performed. Material and methods: The study objectives were to explore the time interval from the treatment starting date to the date of the first radiographic control (TFRC) and evaluate the proportion of patients who achieved ≥ 20% tumor regression within the first 8 weeks of first-line therapy, in the real-world settings. Results: The medians of TFRC in all individual participating centers were > 12 weeks (range 14.0–36.4 weeks). TFRC ≤ 8 weeks was reported for only 3% of patients in the cohort with first-line therapy, and there were only 3 patients (1%) who achieved tumor regression of ≥ 20% by day 60 (8.6 weeks). Conclusion: These findings indicate that the basic time parameter of ETS could not realistically be employed in routine oncology care of patients with metastatic colorectal cancer (mCRC) in the Czech Republic, unless there would be a strict request to perform TRFC by week 8 since the initiation of the therapy. In addition, the frequency of objective tumor response to first-line therapy with cetuximab + chemotherapy was evaluated. Based on the relative regression in the sum of diameters of measurable metastatic lesions, unconfirmed partial responses were achieved in 42.4 % and unconfirmed complete response in 8.6% of patients, altogether corresponding to the overall response rate of 51% with first-line therapy. The frequency of responses was higher among patients with left than right sided primary tumors. It seems that the regimen of cetuximab/FOLFOX might be more active in frontline therapy of right sided RAS wild type mCRC than cetuximab/FOLFIRI.
Keywords:
colorectal cancer – Chemotherapy
Autoři:
J. Fínek 1; M. Vočka 2; J. Bauer 2; E. Kubala 3; K. Zycháčková 4; M. Šedivá 5; M. Gharibyar 4; I. Kocáková 6; E. Čmuchařová 7; M. Vaňková 8
Působiště autorů:
Department of Oncology and Radiotherapy, Faculty of Medicine in Pilsen, University Hospital in Pilsen, Charles University, Czech Republic
1; Department of Oncology, First Faculty of Medicine, Charles University, and General University Hospital in Prague, Czech Republic
2; Department of Oncology and Radiotherapy, Faculty of Medicine in Hradec Králové and University Hospital Hradec Králové, Czech Republic
3; Department of Oncology, Baťa Hospital Zlín, Czech Republic
4; Institute of Radiation Oncology, Bulovka University Hospital, Czech Republic
5; Department of Comprehensive Cancer Care, Masaryk Memorial Cancer Institute, and Faculty of Medicine, Masaryk University, Czech Republic
6; Oncology Department, Masaryk Hospital, Ústí nad Labem, Czech Republic
7; Oncology Clinic, Hospital AGEL Karviná, Czech Republic
8
Vyšlo v časopise:
Klin Onkol 2024; 38(2): 110-117
Kategorie:
Původní práce
doi:
https://doi.org/10.48095/ccko2024110
Souhrn
Východiska: S cílem prokázat proveditelnost implementace konceptu časné nádorové regrese (early tumor shrinkage – ETS) do každodenní klinické praxe v ČR byla provedena neintervenční, multicentrická, jednoramenná, prospektivní studie v reálné klinické praxi. Materiál a metody: Cílem studie bylo sledovat časový interval od data zahájení léčby do data prvního radiologického hodnocení (the first radiographic control – TFRC) a vyhodnotit podíl pacientů, kteří dosáhli ≥ 20 % regrese nádoru během prvních 8 týdnů léčby první linie, a to v reálné klinické praxi. Výsledky: Medián TFRC ve všech jednotlivých zúčastněných centrech byl > 12 týdnů (rozmezí 14,0–36,4 týdnů). TFRC ≤ 8 týdnů byla hlášena pouze u 3 % pacientů v kohortě s první linií léčby a pouze 3 pacienti (1 %) dosáhli regrese nádoru ≥ 20 % do 60. dne (8,6 týdne). Závěr: Tato zjištění naznačují, že základní časový parametr ETS by v rutinní onkologické praxi u pacientů s mCRC v ČR nemohl být reálně využit, pokud by nebyl striktní požadavek na provedení TFRC do 8. týdne od zahájení terapie. Dále byla hodnocena četnost objektivní odpovědi nádoru na první linii léčby cetuximabem + chemoterapií. Na základě relativní regrese součtu průměrů měřitelných metastatických lézí bylo nepotvrzené částečné odpovědi dosaženo u 42,4 % a nepotvrzené úplné odpovědi u 8,6 % pacientů, což dohromady odpovídá celkové četnosti odpovědí 51 % při léčbě první linie. Četnost odpovědí byla vyšší u pacientů s levostrannými než pravostrannými primárními nádory. Zdá se, že režim cetuximab/FOLFOX by mohl být v první linii léčby pravostranného „RAS wild type“ mCRC účinnější než režim cetuximab/FOLFIRI.
Klíčová slova:
kolorektální karcinom – chemoterapie
The evaluation of efficacy of antitumor therapy in solid tumors has been based on an objective change in tumor size. In daily clinical practice, the observation of a tumor response reassures the patient and the oncologist that the selected therapy is active in the malignant disease.
Different criteria have been developed for the evaluation of the change in the tumor size related to the prescribed therapy, which in general defined four basic categories of response: complete (CR) and partial (PR) responses, and stabilization (SD) and progression (PD) of the disease. For a long time, only CR and PR were recognized as a ‘positive’ treatment response. Since the eighties of 20th century, the objective response rate (ORR) as a sum of proportions of CR and PR was used as a surrogate endpoint to overall survival in clinical trials.
Nevertheless, with the introduction of biological anticancer therapy, it has become obvious, that ORR does not strictly correlate with either overall or progression-free survival, and even patients achieving only stable disease, as the best therapeutic response, can ultimately benefit from the therapy, with respect to prolonged survival. However, biological therapies are expensive. Therefore, clinicians have struggled to find other predictive markers that could identify subgroups of patients who would profit most from the biological therapies.
The radiographically confirmed early tumor shrinkage (ETS) has been identified in a series of clinical trials in metastatic colorectal cancer as a valuable marker for prediction of the best objective response and overall survival [1–20]. ETS has been defined as a minimum of ≥ 10% size reduction in the sum of diameters of target lesions achieved by week 6 from the initiation of second-line systemic therapy [5,19], and ≥ 20% size reduction achieved by week 8 from the initiation of first-line therapy [4,8,14,18,20].
Whilst significance of this marker has been confirmed in post-hoc analyses of randomized controlled clinical trials, there is almost no experience with the implementation of this strictly defined marker in daily clinical practice [21–24]. With the aim to show the feasibility of the ETS concept implementation in daily clinical practice, we decided to organize a local, real-world study with the acronym RESECT.
Material and methods
Study design and patients
RESECT was a non-interventional, multicentric, single arm, prospective study covering 17 centers with comprehensive oncology care in the Czech Republic. Patients were recruited by investigators during routine visits in the participating oncology centers. Cetuximab naïve patients aged ≥18 years, with histologically confirmed adenocarcinoma of the colon or rectum, with a wild type of RAS gene, were eligible for this trial.
Patients with planned cetuximab therapy were registered in the study under a specific individual numerical code, which was constructed from serial numbers of the center, the responsible physician in the respective center and the patient treated in the respective center. Thus, a unique subject number was assigned to each subject at inclusion and served as the subject’s identifier in the study as well in the study database. On the Case Report Forms or other documents submitted to the Sponsor, subjects could be identified by their assigned identification numbers only. Only authorized persons had access to identifiable personal details, if required for data verification.
Patients’ therapy as well as clinical and laboratory examinations and their timing were at the discretion of the respective physicians/investigators in the participating centers.
The study protocol together with Informed Consent Form, was submitted to, and finally approved by an Independent Ethics Committee. All patients signed informed consent before initiation of the study therapy.
There were no pre-specified terms for the observation period, except the dates for the first and last patient included in the study and the date of the end of data collection. There was no formal monitoring of the study.
Study objectives
The primary study objective was to explore the between-center and between-patient variability in a time interval from the treatment start date to the date of the first radiographic control (TFRC), with regard to the predefined time interval of 6–12 weeks for the evaluation of early tumor shrinkage. Evaluations were performed both in the full study population and in the subset of patients with first-line therapy only. Secondary objectives included proportion of patients with tumor regression of ≥ 20% by week 8 since the initiation of the therapy. The depth of response (DpR), as an additional study endpoint, was evaluated, too. It was defined as the maximum relative tumor regression achieved during the treatment, in comparison to the baseline measurable tumor size. For the purpose of this study, it was evaluated as a categorial variable based on the split into relative regression categories of < 10%, ≥ 10%, ≥ 20%, ≥ 30%, ≥ 50% and 100%. In addition to that, objective response rates were evaluated. All these evaluations were planned for the subset of patients with first-line therapy only.
Data sources, measurements and management
The regular clinical/hospital records of eligible patients were the basic source of information with regard to demography, history, baseline status, therapy and its outcomes, follow-ups and related changes in tumor load and health status. For detailed radiography evaluation of the tumor load and its changes, either CT or MRI scans were used.
Diagnostic, treatment and follow- -up data of individual patients included in the study were registered through the study electronic Case Report Forms (eCRF). These were filled-in by the respective physician/investigator from each participating center. There was no independent evaluation of the tumor load and its changes. No strict requirements for frequency and/or time intervals of assessments were set-up for this non-interventional study.
The data were entered into a validated electronic database and processed by an independent data managing body. Quality control was performed before the electronic database was locked and released for statistical analysis as documented in the Data Management Report. Database lock occurred once data completeness procedures had been finished.
There was no adverse event reporting in the period from the start of the study in May 2013 up to the implementation of the protocol amendment in May 2015. Since its implementation, each suspected adverse event/reaction occurring during the study, whether serious or not, had to be recorded in the eCRF, including its description, severity, duration (onset and resolution dates), causal relationship (i.e., confirmation that the adverse reaction is suspected to be reasonably related to the study treatment).
Statistics
The analysis of time to TFRC was restricted to patients with a known KRAS/RAS status and a follow-up period of at least 6 weeks, who had used the same methods for tumor size measurement at baseline and during the therapy, had no missing baseline tumor measurements and the baseline tumor measurements of lymph nodes were evaluated with other than ultrasound imaging methods.
Regarding TFRC, descriptive statistics including means (standard deviation and 95% confidence interval (CI), medians and interquartile range (IQR) were used for each of the participating centers. The between-center variance of time to TFRC was evaluated for 6 centers with ≥ 20 enrolled patients, using the Kruskal-Wallis tests.
Proportions of patients achieving tumor regression of ≥ 10%, ≥ 20%, ≥ 30%, ≥ 50% and 100% were calculated, without any formal statistical analysis. For patients achieving each respective tumor regression percentage, the time to achieve that percentage regression was also calculated (mean, SD, 95% CI; median, IQR). Correlations between the time to achieve and the size of the respective tumor shrinkage were evaluated using Spearman’s correlation.
No survival analyses were planned in this non-interventional, real world study.
Results
The RESECT trial was initiated in April 2013. The last patient entered the study at the end of November 2017. The study was closed on February 28, 2019. Altogether, 636 patients with metastatic colorectal cancer were enrolled into the study, 435 (68%) indicated for first-line therapy, 174 (28%) for second-line therapy and 27 (4%) for third-line therapy. There were 427 (67.1%) males and 209 (32,9%) females in the full study population.
Left sided tumors represented the majority of cases (81%), while right sided tumors were much less frequent (19%) and were slightly more frequent among women than in men (26% and 16% respectively). The median age of all enrolled patients was 63 years (range 21–83) and was not different between men and women (65 and 64 years, respectively), nor between left-sided and right-sided primary tumors (65 years for both sides).
Given that no survival analyses were planned, we evaluated only time of patients’ follow-up during the study. The median of the study follow-up duration in the cohort of 304 patients with first-line therapy was 233 days (range 23–1 527), with only 80 patients (26.3%) being followed for > 1 year, 14 patients (4.6%) for > 2 years and 5 patients (1.6%) for > 3 years.

TFRC evaluation (primary endpoint)
The full study population
Out of 636 patients in the intention to treat population, TFRC was thought to be non-evaluable for 152 (23.9%) of them. Different methods were used for tumor size measurement at baseline and during therapy (N = 97; 15.3%), the baseline tumor size was evaluated only with ultrasound (N = 25; 3.9%), missing baseline tumor size measurements (N = 19; 3.0%), follow-up period < 6 weeks (N = 9; 1.4%), and an unknown RAS status (N = 2; 0.3%) were further reasons for excluding patients from the analyses. Therefore, 484 patients (304 receiving first-line, 155 receiving second-line and 25 receiving third-line therapy) were eligible for the evaluation of the time to TFRC.
The mean TFRC was 121 days (17.3 weeks), with the median of 107 days (15.3 weeks) and a range of 6–457 days (Tab 1). A TFRC ≤ 60 days (8.6 weeks) was reported in only 9.8% of patients and a TFRC ≤ 90 days (12.9 weeks) in 19.2% of patients.

First-line therapy subset of patients
There were 304 out of 435 (69.9%) patients in the first-line therapy subset, who were eligible for TFRC evaluation. The mean TFRC was 124.9 days (17.8 weeks) with 95% CI 119.6–130.2 days; median TFRC was 118.5 days (16.9 weeks), interquartile range (IQR) 98–143.5 days (14–20.5 weeks) and the range 65–221 days (9.3–31.6 weeks) (tab. 1). TFRC ≤ 8 weeks was reported for only 9 patients (3%).
The medians of TFRC in all individual participating centers were > 12 weeks (range 14.0–36.4 weeks). Analyses of inter-center differences in TFRC were performed, comparing TFRCs of six centers, which provided ≥ 20 patients receiving first-line therapy. The median TFRC in any of the six tested centers was substantially > 8 weeks (medians in the range of 15.0–18.9 weeks) and there was a statistically significant difference between the individual centers (P = 0.0079; ANOVA Kruskal-Wallis test) (Fig. 1).
Early tumor shrinkage and DpR in the first-line therapy subset
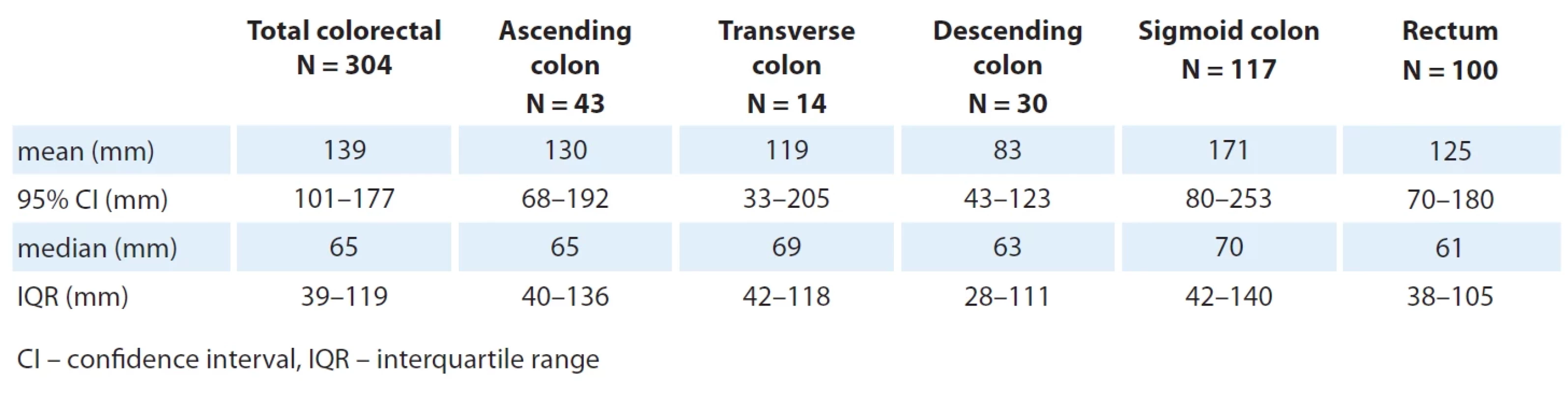
Baseline tumor size
A baseline tumor size was defined as the sum of the longest diameters of all measurable tumor lesions. The results of these measurements are presented in Tab. 2. There were no statistically significant differences in the baseline tumor size with regard to location of the primary tumor (P = 0.4505; ANOVA Kruskal-Wallis test) and between left-sided and right-sided tumors (median 65 mm for both sides; P = 0.5821; two-sided ANOVA Wilcoxon two-sample test), as well as between males and females (medians 67 and 65 mm, respectively; P = 0.7960; two-sided ANOVA Wilcoxon two-sample test).
Early tumor shrinkage
In the first-line therapy cohort, 181 out of 304 (59.5%) evaluable patients had regression of ≥ 20% confirmed by radiologic imaging at any time (Tab. 3). However, based on the time of TFRC, such regression was confirmed only in 3 patients (1%) by day 60 (8.6 weeks), and in 28 patients (9.2%) for whom this was confirmed by day 90 (12.6 weeks). In view of the extremely low number of patients with data fulfilling the criteria for ETS, the analysis of correlation between ETS and DpR was not performed.


Relative tumor regression (DpR)
DpR achieved during the treatment was evaluated in the cohort of 304 patients with first-line therapy. The results are summarized in Tab. 3. Regression of at least 10% was achieved by 69% of patients. Regression of ≥ 20% (critical value for the ETS) was achieved in 59,5% of patients, regression of ≥ 30% (equivalent to ORR based on RECIST1.1) was achieved in 51% of patients and regression of 100% (equivalent to CR by RECIST1.1) was achieved in 8,6% of patients.
The median time to the first occurrence of regression of ≥ 10% was 124 days (IQR 101–153). As it could be expected, the median time to the first occurrence of a specific level of tumor regression increased, while the percentage of patients decreased when a stronger level of regression (≥ 50% and 100%) was to be achieved (Tab. 3, Fig. 2).
Unconfirmed objective tumor responses
Out of 304 patients in the first-line therapy cohort 214 (70%) were males and 90 (30%) were females. Left sided primary tumors represented the majority of all cases in the cohort (81.2%) compared to right sided tumors (18.8%). The treatment regimen of cetuximab + FOLFOX was prescribed to 43% and the regimen of cetuximab + FOLFIRI to 40% of patients in this cohort. The remaining 17% of patients were prescribed either with regimens of cetuximab + irinotecan (2%) or cetuximab + fluoropyrimidines only (15%). Treatment responses were assessed by the prescribing physicians/investigators, and there was neither any formal independent validation nor a formal statistical analysis.
The ORR in the whole evaluable cohort was 52%, with 11.5% of complete responses. These figures are quite similar to the figures estimated on the basis of evaluations of relative tumor regression.
The frequency of responses was higher among patients with left than right sided primary tumors and slightly higher among males than females (Tab. 4). Based on ORR estimates, it seems that regimens of cetuximab/FOLFOX could be more active than regimens of cetuximab/FOLFIRI in the frontline therapy of RAS-wt mCRC. Combinations of cetuximab with irinotecan or fluoropyrimidines alone seem to be clearly less effective as first-line therapies (Tab. 5). Both overall ORR and complete response rate (CR) were more favorable with cetuximab + FOLFOX than with cetuximab + FOLFIRI, both in left and right sided tumors (Tab. 6).
During the study follow-up period, 229 (75.3%) out of 304 patients progressed or died, with 73% of them having left sided tumors and 86.0% of them having right sided tumors. Out of 56 patients who died during the study follow-up period, 44 (17.8%) of them had left sided tumors and 12 (21.1%) of them had right sided tumors. These findings suggest a less favorable prognosis with higher mortality for patients with right sided tumors. Out of 229 patients with progression or death during the study follow-up period, only 60 (26.2%) were followed for > 1 year, and out of 56 patients who died during the study follow--up period, only 7 (12.5%) were followed for > 1 year. Based on these findings, we can mechanistically assume that in our cohort of 304 patients with first-line therapy, the majority of patients (169/229; 73.8%) had progressed during the first year of the follow-up.



Safety
The safety population in the RESECT study comprised 478 patients, who received at least one dose of cetuximab, 374 being patients with first-line, 93 patients with second-line and 11 with third-line therapy. Altogether there were 276 adverse events (AEs) reported, 221 from first-line, 41 from the second-line and 8 from the third-line patient cohorts. The majority of all AEs was reported as G1/2 (91.6%), while AEs with G ≥ 3 represented 8.4% of all reported AEs, including single cases of G5 AEs (0.2%). The adverse events were reported more frequently in male than female patients (48.5 vs. 33.0%, respectively). The same was observed with respect to cetuximab-related AEs (32.3 vs. 17.8%).
The most frequently reported AE was skin toxicity comprising erythema, exanthema, acneiform rash, dry skin, skin rhagade and paronychia (38.7%; G3/4 3.1%), followed by neurotoxicity, comprising paresthesia and peripheral neuropathy (7.3%; G3 0,4%), diarrhea (6.3%; G3/4 1.2%) and neutropenia (5.4%; G3/4 3.4%, G5 0.2%). Cetuximab-related infusion reactions were reported in seven cases (1.5%), with only one such reaction reported as G3 (0.2%).
Temporary or permanent discontinuations of therapy due to AEs were reported in 20 cases (4.2%), temporary discontinuations in 13 cases (2.7%), permanent discontinuations in 7 cases (1.5%). Cetuximab was discontinued in all reported cases during the study. Dose reductions of chemotherapy due to AEs were reported in 14 cases (2.9%); in 9 cases, the reported AEs were considered cetuximab-related (1.9%). There was no reduction of cetuximab dose. Safety analyses in the RESECT study provided no new safety signals.
Discussion
Given that ETS had appeared as a predictor of higher sensitivity of mCRC to systemic anticancer therapy as well as of better treatment outcomes and prognosis, particularly for first-line therapy, we were interested whether this parameter could be used in routine clinical practice in the Czech Republic. The definition of ETS in the RESECT study was the same as in controlled randomized trials with cetuximab in mCRC: relative regression of measurable tumor size by ≥ 20% within the first 8 weeks of therapy.
We aimed to evaluate the time to TFRC as a primary endpoint to see what proportion of RAS-wt mCRC patients will fulfill the time criterion of the ETS concept in a real world setting.
Within the population of all evaluable patients (N = 484), the median time to TFRC in all participating centers exceeded the threshold value of 8 weeks, predefined for ETS evaluations, and was even higher than the time for regular follow-up visits as recommended by European Society for Medical Oncology [25] and local guidelines (2–3 months) [26]. The proportion of patients with TFRC carried out ≤ 60 days after therapy initiation was substantially lower (9.8%) than expected. On top of that, there were significant differences in TFRC between individual participating centers. In the cohort of 304 patients with first-line therapy, a median TFRC of 17 weeks (IQR 14–20 weeks) was observed, and the proportion of patients with TFRC ≤ 8 weeks was 3% only. These findings indicate that the basic time parameter of ETS could not realistically be employed in routine oncology care of patients with mCRC in the Czech Republic, unless there would be a strict request to perform TFRC by week 8 after therapy initiation.
As the second important parameter, relative regression from the baseline of measurable tumor size was evaluated in the cohort of patients with first-line therapy. Regression by ≥ 20% was achieved in 60% of patients; however, it was reported as achieved within the first 8 week of therapy for 2 patients (1.1%) only, probably due the low rate of patients receiving their TFRC 8 weeks after the start of the therapy. As the median of TFRC in this cohort of patients was almost 17 weeks and the proportion of patients with TFRC ≤ 8 weeks was 3% only, we were not able to estimate the real proportion of patients who achieved regression by ≥ 20% by week 8.
With extremely low number of patients with data fulfilling the criteria of ETS, analysis of the correlation between ETS and DpR would be invalid and was not performed.
The RESECT study was a non-interventional prospective clinical trial, in which timing of any clinical, radiographic and laboratory examinations was at the discretion of the respective physician/investigator from the participating center. This was a major limitation, which made more precise evaluation of the ETS concept impossible.
On the other hand, we were able to evaluate treatment response, based on the data of relative tumor regression on one side and investigators reported objective responses on the other side. Given that ORR was not the primary endpoint in this non-interventional study, confirmation of responses was not required.
Based on the relative regression in the sum of diameters of measurable metastatic lesions, unconfirmed partial responses were achieved in 42.4% and unconfirmed complete responses in 8.6% of patients, altogether corresponding to ORR of 51% with first-line therapy. Based on investigator-reported objective response, the overall ORR as well as frequency of complete responses were similar (52 and 11.5%, respectively). The frequency of responses was higher among patients with left- than those with right-sided primary tumors.
Conclusion
It seems that the regimen of cetuximab/FOLFOX might be more active than the regimen of cetuximab/FOLFIRI in frontline therapy of RAS-wt mCRC, particularly in right-sided tumors. All these findings are consistent with the data from randomized clinical trials and their meta-analyses in patients with mCRC [1,10,16,25,27].
The safety analysis of the RESECT study provided no new safety signals. The safety profile of cetuximab was similar to that reported in other clinical trials, and there was just a single case of therapy-related death reported during the study. However, the reported frequencies of individual adverse reactions seemed to be lower than in controlled clinical trials. Potential explanation could be underreporting, as generally recognized in non-interventional studies and/or postmarketing pharmacovigilance and spontaneous AE reporting.
Financial support
This research was financially supported by Merck spol. s r. o., Praha, Czech Republic, an affiliate of Merck KGaA, Darmstadt, Germany.
Zdroje
1. Arnold D, Lueza B, Douillard JY et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol 2017; 28 (8): 2932–2942. doi: 10.1093/annonc/mdx175.
2. Bouchahda M, Boige V, Smith DM et al. Impact of early tumor response on prognostic of patients with unresectable liver metastases from wt-KRAS colorectal cancer (LM-CRC) treated with hepatic artery infusion of irinotecan, 5-fluorouracil and oxaliplatin plus intravenous cetuximab after failure of systemic chemotherapy (European Phase II OPTILIV). J Clin Oncol 2016; 34 (15 Suppl): e15036. doi: 10.1200/JCO.2016.34.15_suppl.e15036.
3. Carrato A, Abad A, Massuti B et al. First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: a randomised, phase II trial (PLANET-TTD). Eur J Cancer 2017; 81: 191–202. doi: 10.1016/j.ejca.2017.04.024.
4. Cremolini C, Loupakis F, Antoniotti C et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol 2015; 26 (6): 1188–1194. doi: 10.1093/annonc/mdv112.
5. De Roock W, Piessevaux H, De Schutter J et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 2008; 19 (3): 508–515. doi: 10.1093/annonc/mdm496.
6. Douillard JY, Siena S, Peters M et al. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer 2015; 51 (10): 1231–1242. doi: 10.1016/ j.ejca.2015.03.026.
7. Giessen C, Laubender RP, Fisher von Weikersthal L et al. Early tumor shrinkage in metastatic colorectal cancer: retrospective analysis from an irinotecan-based randomized first-line trial. Cancer Sci 2013; 104 (6): 718–724. doi: 10.1111/cas.12148.
8. Heinemann V, Stintzing S, Modest DP et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 2015; 51 (14): 1927–1936. doi: 10.1016/j.ejca.2015.06.116.
9. Heun JM, Grothey A, Branda ME et al. Tumor status at 12 weeks predicts survival in advanced colorectal cancer: findings from NCCTG N9741. Oncologist 2011; 16 (6): 859–867. doi: 10.1634/theoncologist.2011-0064.
10. Holch JW, Ricard I, Stintzing S et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 2017; 70: 87–98. doi: 10.1016/j.ejca.2016. 10.007.
11. Cheng AL, Cornelio G, Shen L et al. Efficacy, tolerability, and biomarker analyses of once-every-2-weeks cetuximab plus first line FOLFOX or FOLFIRI in patients with KRAS or all RAS wild-type metastatic colorectal cancer: the phase 2 APEC study. Clin Colorectal Cancer 2017; 16 (2): e73–e88. doi: 10.1016/j.clcc.2016.08.005.
12. Ichante J, Adenis A, Malka D et al. Impact of early tumor shrinkage on long-term outcome in metastatic colorectal cancer (mCRC) treated with 5FU plus irinotecan plus leucovorin (FOLFIRI) or capecitabine plus irinotecan XELIRI plus bevacizumab. J Clin Oncol 2011; 29: e14041. doi: 10.1200/jco.2011.29.15_suppl.e14041.
13. Ito M, Kusaba H, Mukaide S et sl. Early tumor shrinkage indicates a favorable response to bevacizumab-based first-line chemotherapy for metastatic colorectal cancer. Anticancer Drugs 2017; 28 (10): 1166–1173. doi: 10.1097/CAD.0000000000000562.
14. Köhne CH, Karthaus M, Mineur L et al. Impact of primary tumour location and early tumour shrinkage on outcomes in patients with RAS wild-type metastatic colorectal cancer following first line FOLFIRI plus panitumumab. Drugs R D 2019; 19 (3): 267–275. doi: 10.1007/s40268-019-0278-8.
15. Loupakis F, Yang D, Yau L et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015; 107 (3): dju427. doi: 10.1093/jnci/dju427.
16. Loupakis F, Cremolini C, Masi G et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014; 371 (17): 1609–1618. doi: 10.1056/NEJMoa1403108.
17. Modest DP, Laubender RP, Stintzing S et al. Early tumor shrinkage in patients with metastatic colorectal cancer receiving first-line treatment with cetuximab combined with either CAPIRI or CAPOX: an analysis of the German AIO KRK 0104 trial. Acta Oncol 2013; 52 (5): 956–962. doi: 10.3109/0284186X.2012.752580.
18. Modest DP, Stintzing S, Fisher von Weikersthal L et al. Relation of early tumor shrinkage (ETS) observed in first-line treatment to efficacy parameters of subsequent treatment in FIRE-3 (AIOKRK0306). Int J Cancer 2017; 140 (8): 1918–1925. doi: 10.1002/ijc.30592.
19. Piessevaux H, Buyse M, De Rock W et al. Radiological tumor size decrease at week 6 is a potent predictor of outcome in chemorefractory metastatic colorectal cancer treated with cetuximab (BOND trial). Ann Oncol 2009; 20 (8): 1375–1382. doi: 10.1093/annonc/mdp011.
20. Piessevaux H, Buyse M, Schilchting M et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2013; 31 (30): 3764–3775. doi: 10.1200/JCO.2012.42.8532.
21. Rivera F, Karthaus M, Hecht JR et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis 2017; 32 (8): 1179–1190. doi: 10.1007/s00384-017- 2800-1.
22. Suzuki C, Blomqvist L, Sundin A et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol 2012; 23 (4): 948–954. doi: 10.1093/annonc/mdr350.
23. Sommeijer D, Shi Q, Saad ED et al. Early predictors of overall survival (OS) in patients (pts) on 1st-line chemotherapy (CT) for metastatic colorectal cancer (mCRC): an ARCAD study with individual patient data (IPD) on 10,962 pts. J Clin Oncol 2014; 32 (15 Suppl): 3538. doi: 10.1200/jco.2014.32.15_suppl.3538.
24. Taieb J, Rivera F, Siena S et al. Exploratory analyses assessing the impact of early tumour shrinkage and depth of response on survival outcomes in patients with RAS wild-type metastatic colorectal cancer receiving treatment in three randomised panitumumab trials. J Cancer Res Clin Oncol 2018; 144 (2): 321–335. doi: 10.1007/s00432-017-2534-z.
25. Tejpar S, Stintzing S, Ciardiello F et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2017; 3 (2): 194–201. doi: 10.1001/jamaoncol.2016.3797.
26. Van Cutsem E, Cervantes A, Adam R et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27 (8): 1386–1422. doi: 10.1093/annonc/mdw235.
27. Venook AP, Niedzwiecki D, Innocenti F et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405. J Clin Oncol 2016; 34 (Suppl 15): 3504. doi: 10.1200/jco.2016.34.15_suppl.3504.
Štítky
Dětská onkologie Chirurgie všeobecná OnkologieČlánek vyšel v časopise
Klinická onkologie

2024 Číslo 2
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Léčba akutní pooperační bolesti z pohledu ortopeda
- Nejasný stín na plicích – kazuistika
- Vysoká hladina PSA a její rychlý nárůst jsou nepříznivými prognostickými faktory u karcinomu prostaty
- Proces hojení ran krok za krokem a co ho může zkomplikovat
Nejčtenější v tomto čísle
- Možnosti zavedení časné nádorové regrese jako potenciálního prediktivního markeru do každodenní klinické praxe u pacientů s metastatickým kolorektálním karcinomem RAS divokého typu léčených cetuximabem – neintervenční observační studie
- Neuroonkologie jako perspektivní oblast medicíny
- Význam aberantní metylace DNA pro diagnostiku a terapii nádorových onemocnění
- Faktory ovplyvňujúce prežívanie pacientov a vývoj GvHD po alogénnej transplantácii krvotvorných buniek od HLA-identických súrodencov – skúsenosť jedného centra
